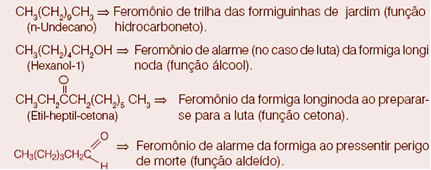
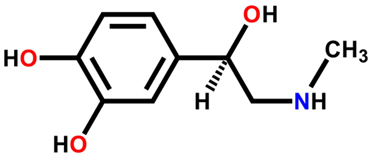
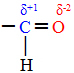question 1
(UFAM) The intermolecular dehydration reaction of an organic compound in the presence of 140 sulfuric acidOC, produced a highly flammable liquid, less dense than water, with strong anesthetic action and sufficient non-polarity for the extraction of oils and fats. The compound and the liquid that fit these characteristics are, respectively:
a) Propanol and ethyl acetate
b) Methanol and methoxymethane
c) Acetic acid and ethyl ethanoate
d) Acetone and ethoxymethane
e) Ethanol and ethoxyethane
question 2
(FGV-SP) When ethanol is brought into contact with sulfuric acid, hot, a dehydration reaction occurs, and the products formed are related to the reaction temperature. Intramolecular dehydration occurs at 170ºC and intermolecular dehydration at 140ºC. The products of intramolecular and intermolecular dehydration of ethanol are, respectively,
a) ethane and ethoxyethene.
b) ethene and ethoxyethane.
c) ethoxyethene and ethene.
d) ethoxyethane and ethene.
e) ethoxyethene and ethane.
More questionsWatch our video class to learn a little about the history of Englishwoman Margaret Thatcher (1925-2013). Also check out our channel for other information about the history of women.
Considering the dynamism of the Portuguese language, one cannot deny the importance of studying linguistic varieties, a theme recurrently raised in Enem. In this video class, we will analyze, in practice, how questions about this content are presented. Check out!