Metals are elements characterized by brightness, strength, thermal and electrical conductivity. They are applicable in virtually all industrial processes, being present in metallic alloys used in the manufacture of tools, jewelry and coins and in chemical processes such as redox, responsible for the operation of stacks and batteries.
In the Periodic Table, they are classified into:
semimetals
transition metals
alkali metals
alkaline earth metals
In the steel industry, they are separated into:
ferrous metals
non-ferrous metals
heavy metals
Read too: What are the new elements of the periodic table?
Metal characteristics
They are, for the most part, excellent conductors of heat and electrical energy.
They have a glossy appearance.
have high density.
They are electropositive elements (they tend to form cations — positive charges).
With the exception of the Mercury, they are elements with a high melting point and solid at room temperature.
They have mechanical strength (tenacity).
They are malleable (can be molded without breaking).
Do not stop now... There's more after the advertising ;)
Periodic Table Metals
alkaline metals
Are the elements of first column of the periodic table, family 1A. They are called alkali metals because, when mixed with water, they form hydroxides, that is, an alkaline (basic) solution.
You elements that belong to this group are:
Lithium (li)
Sodium (At)
Potassium (K)
Rubidium (Rb)
Cesium (Cs)
Francium (Fr)
The electronic configuration of alkali metals in neutral (uncharged/ionized) state only presents an electron in the last shell (valence layer). O atomic ray and the reactivity of these metals increases from top to bottom (observing the Periodic table). They have a low melting point compared to other metals, are malleable and not very dense, have a matte aspect due to the high oxidation potential.
See too:What are the properties of matter?
alkaline earth metals
These are elements that appear in the second column of the periodic table, family 2A. are called alkaline earth by the tendency to form solutions of a basic (alkaline) character, and by earth because, before the 19th century, the oxides formed by these substances were designated that way. This term was used to designate insoluble non-metals that did not undergo mutation when heated, common characteristics of group 2 elements. Later, in light of new research and experiments, it was discovered that the “earth” elements were actually oxides formed by metals.
They are malleable metals, with low density, have two electrons in its outermost shell, are found, under normal conditions of temperature and pressure, in solid state. Belong to this group:
Beryllium (Be)
Magnesium (Mg)
Calcium (Here)
Strontium (Mr)
Barium (Ba)
Radio (Frog)
transition metals
make up the group B of the Periodic Table, and are located between the alkaline earth metals and the ametals. They were defined by IUPAC as elements that have an incomplete energéticad’ energetic sublayer. The term "transition metals" refers to transition from group 2 to 13 in the Periodic Table and the increasing addition of electrons in the ‘d’ orbital.
The transition metal group is composed of dense elements, with high melting and boiling points, and less reactive than group 1 and 2 metals (Family 1A and 2A). Among the transition metals are tungsten, which is the element with the highest melting point (3422 °C), and mercury (Hg), which is a metal that is liquid under normal conditions of temperature and pressure, its melting point is (-38.83 °C).
Transition metals have various oxidation states (NOX variable) and form colored substances.
Internal transition elements
Internal transition elements they are:
Lanthanides: series composed of elements that have from 57 to 71 protons and that, with the exception of promethium, are natural.
Actinides: group formed by the elements ranging from 89 to 103 in atomic number and which, for the most part, are synthetic. All of this group are radioactive with short half-life.
The elements of these two groups have a high melting point and are all solid at room temperature.
Semi metals
You semimetals there are, in all, seven elements:
Boron (B)
Silicon (Si)
Germanium (Ge)
Arsenic (As)
Antimony (Mon)
Tellurium (Te)
Polonium (Po)
This classification is given to elements that have physicochemical characteristics relevant to metals and also others relevant to non-metallic elements. Semimetals have a shine, are brittle compounds, capable of forming cations and also anions (depending on the condition), and are intermediate conductors.
Types of Metals
Ferrous: substances that contain iron in their composition. They are metal alloys which contain iron as a common element, but can be formulated with different concentrations of carbon, for example, or forged with different techniques, thus having products with different physical properties.
Non-ferrous: alloys formed by other types of metals, such as aluminum, copper, nickel, zinc, titanium.
Heavy metals: lead, nickel, zinc, mercury. They are reactive, toxic and bioaccumulative metals. They are used in batteries, lamps, ammunition for military artifacts, among others.
Chemical bonds of metals
Metals are elements electropositive, that is, they have a tendency to lose electrons in a chemical bond. When a metal interacts with a non-metal, it perform an ionic bond due to the difference in electronegativity. The other bonding possibility for metals is metal bonds.
At metal connections occur between metals, with partial release of electrons and the formation of a cloud or sea of electrons around the atoms, which guarantees the metal parts the properties of conductivity electrical and malleability.
See too: Conductors and insulators – what are the differences?
Obtaining metals
Most metals are found naturally in rocks and minerals associated with other elements. There are several techniques for refining metals, such as through electrolysis and chemical reactions to separate compounds.
In the case of metals, the two most used techniques on an industrial scale to obtain a certain degree of purity in a metal are: difference of density and magnetic susceptibility (tendency that the metal has to react to a magnetic field).

solved exercises
Question 1 - (Enem) Cadmium, present in batteries, can reach the ground when these materials are irregularly disposed of in the environment or when they are incinerated. Unlike the metallic form, Cd2+ ions are extremely dangerous for the body as they can replace Ca2+ ions, causing a degenerative disease in the bones, making them very porous and causing severe pain in the bones. joints. They can also inhibit enzymes activated by the Zn2+ cation, which are extremely important for the functioning of the kidneys. The figure shows the variation in the radius of some metals and their respective cations.
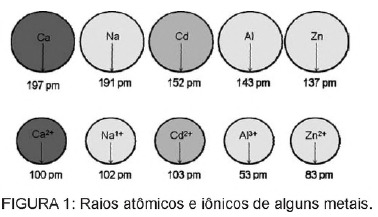
Based on the text, the toxicity of cadmium in its ionic form is a consequence of this element
A) present low ionization energy, which favors the formation of the ion and facilitates its binding to other compounds.
B) have a tendency to act in biological processes mediated by metallic cations with charges ranging from +1 to +3.
C) have a radius and charge relatively close to metal ions that act on biological processes, causing interference in these processes.
D) have a large ionic radius, allowing it to interfere in biological processes in which smaller ions normally participate.
E) have a +2 charge, which allows it to interfere with biological processes in which, normally, ions with lower charges participate.
Resolution
Alternative C. Cadmium is an element similar to the metallic ions that we naturally have in the body. In addition to being bioaccumulative, cadmium, having this similarity to metallic groups, can bind to sulfhydryl groups of molecules of protein, thus causing changes in biological processes, such as decalcification, and tissue and red blood cell destruction blood.
Question 2 - For characteristics relevant to metals, tick the incorrect alternative.
A) Most metals are good electrical and thermal conductors.
B) Some metals are susceptible to oxidation and/or reduction.
C) Iron is a resistant and malleable metal, that is, it is easy to mold.
D) Alkaline earth metals have this name “earth” referring to the oxides of these metals, which are dark in color.
E) Metals are elements of varied colors and dull, due to the action of oxidation.
Resolution
Alternative E. Metals are naturally shiny and metallic in color, they can undergo oxidation, which causes changes in the characteristics relevant to the aspect of the part, but this is not a phenomenon that happens in all metals, and, in some cases, oxidation depends on several factors external.
by Laysa Bernardes
Chemistry teacher



