Carbon 14 is a radioactive isotope of carbon that is formed in the Earth's stratosphere when cosmic ray neutrons bombard nitrogen – 14 present in these upper layers of the atmosphere:
717N+ 01n → 614Ç+ 11H
As its symbol above shows, its mass number (the amount of protons and neutrons in the nucleus) is equal to 14.
It ends up entering the natural carbon cycle, as it reacts with oxygen in the air, forming carbon dioxide (CO2). This gas is then incorporated by all plant and animal beings. Vegetables carry out the photosynthesis process, in which they absorb carbon dioxide with carbon 14. Humans and herbivorous animals, on the other hand, feed on these plants and also incorporate carbon 14. Meanwhile, carnivores feed on herbivorous animals and, finally, it ends up being assimilated by all living beings of all trophic levels.
The amount of carbon 14 present in the atmosphere is constant, being equal to about 14 dpm. g-1, this because the speed with which this element forms is the same speed with which it disintegrates.
Plant and animal organisms absorb carbon 14 throughout their life, and its concentration will increasing until the equilibrium mentioned above is established and the amount by mass of carbon 14 becomes equal to that present in the atmosphere. However, when the plant, the human being or the animal dies, the carbon is no longer absorbed and just disintegrates.
With that in mind, scientists use carbon 14 to determine the age of animal and plant fossils or any object that is a by-product of such, such as a piece of wood or cloth.
Do not stop now... There's more after the advertising ;)

The carbon-14 dating technique is used to determine the age of a fossil as shown above.
For this, the half-life period or semi-disintegration period of carbon 14, which is the time it takes for half of its radioactive nuclei to disintegrate, that is, the time it will take for the sample of radioactive material to be reduced by half. The half-life of carbon 14 is approximately 5730 years:
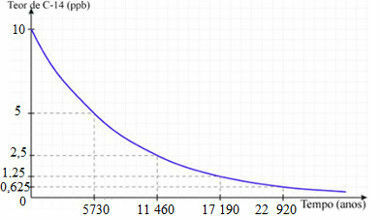
Carbon 14 radioactive decay curve
So, consider an example to see how to determine the age of fossils. Let's say that a fossil of an animal is found and, after analysis, it is found to have a carbon-14 content of 1.25 ppb. So it has 12.5% of the carbon content found in living things. This means that the animal in question died and since then carbon 14 has completed three half lives, which gives a total of17 190 years.That's the age of the fossil!
The carbon-14 dating technique can be safely applied only to objects that are between 100 and 40,000 years old. In the case of dating objects around 100 years old, the amount of radiation emitted will have decreasing very little, and in the case of objects over 40,000 years old, the radiation emitted will be practically equal to zero.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "What is Carbon 14?"; Brazil School. Available in: https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-carbono-14.htm. Accessed on June 28, 2021.
