Enzymes are biological catalysts responsible for increasing the speed of a given chemical reaction. Enzymes are usually proteins, but there are some ribonucleic acids which act as enzymes, being called ribozymes.
In order to speed up a reaction, enzymes must bind to reagents, which are known as substrates. For a long time, this link was believed to occur in a very rigid way, a pattern known as the key-lock. at the moment, However, the model known as induced fitting is accepted., which assumes that slight changes occur in the form of the enzyme as the substrate enters the active site.
Read too: What is metabolism?
What are enzymes?
Enzymes are biomolecules that act as catalysts, that is, they are substances capable of accelerating the speed of chemical reactions that occur in living beings without being consumed during these reactions. Without the action of enzymes, some reactions would be very slow, which would harm metabolism. Enzymes selectively accelerate reactions and are therefore very specific catalysts.
Enzymes are able to speed up a reaction by decreasing the activation energy, that is, they reduce the amount of energy that must be added for a reaction to start.
[publication_omnia]
Is every enzyme a protein?
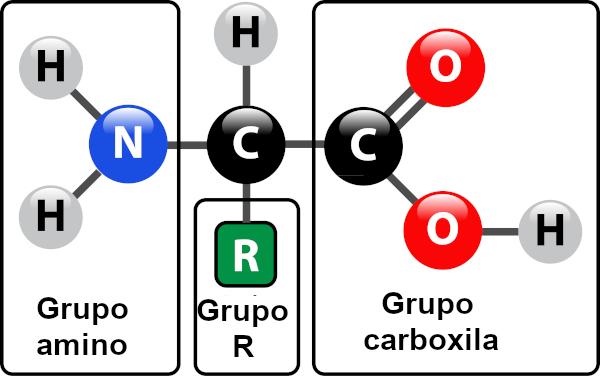
Although they are often defined as biological catalysts of a protein nature, not every enzyme is a protein. There are some RNAs that function like enzymes, called ribozymes. Most enzymes, however, are proteins, being formed, therefore, by amino acids. The amino acid composition of these biomolecules defines the three-dimensional structure it will acquire.
Read too: How do catalyst substances work?
Enzyme-Substrate Complex
It is called the reagent substrate on which an enzyme acts. When an enzyme binds to its substrate, the complex forms enzyme-substrate. This binding takes place in a specific region, called the active site.
When we talk about protein-based enzymes, the active site corresponds to just a few amino acids, with the remainder of the molecule being responsible for determining the configuration of the active site. The shape of the active site as well as the shape of the substrate are related to the specificity of the enzyme, as they must be complementary.
Key-lock model
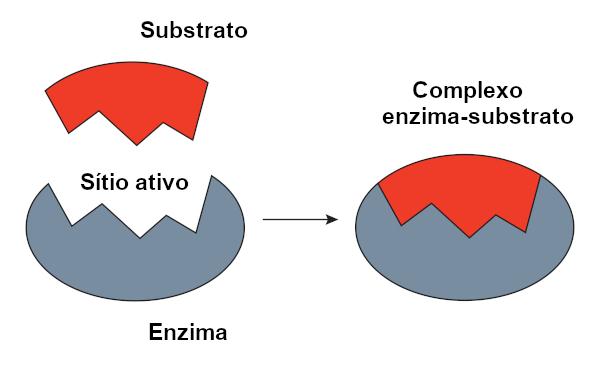
O key-lock model, proposed by Emil Fischer, is widely used to explain the interaction between enzyme and substrate. According to this model, there is a rigid complementarity between the enzyme and the substrate, just like a key and a lock. The enzyme's active site would have a complementary shape to the substrate, which would fit perfectly. Other molecules, therefore, would not have access to this site, which would guarantee the specificity of the enzyme. Just as a key opens only a lock, an enzyme would only bind to a substrate. Today we know, however, that this model is not correct, since enzymes are not rigid structures as previously thought.
Induced fitting model
Currently, the most accepted model to explain the link between an enzyme and its substrate is the one of snap induced, initially proposed by Koshland et al. The active site and substrate do not work rigidly like a key and lock. Research shows that as the substrate enters the active site, the enzyme undergoes a slight modification, which favors the fit between the active site and the substrate. To better understand this model, we can think of the enzyme and substrate interaction as a handshake, which becomes firmer after the first contact.
Cofactors
Most enzymes need auxiliary molecules to carry out their catalytic action, called cofactors. Cofactors can be permanently attached to the enzyme or can be weakly and reversibly attached to the substrate. They also can be inorganic or organic. When cofactors are organic molecules, they are called coenzymes.
Some vitamins act as coenzymes, this being the case, for example, of riboflavin, also known as vitamin B2. As examples of inorganic cofactors, we can mention iron and zinc in their ionic form.
Read too: B-complex vitamins — a group of vitamins that generally act as coenzymes
Enzyme classification
Enzymes can be classified into six groups, using as a criterion the type of reaction they catalyze.
Oxidoreductases: enzymes related to the reactions of oxirreduction.
Transferases: catalyze the transfer of groups from one compound to another.
Hydrolases: catalyze hydrolysis reactions.
Liases: act by adding groups to double bonds or removing groups forming a double bond.
Isomerases: catalyze isomerization reactions.
Links: enzymes that cause the degradation of the molecule of ATP, using the energy released in this reaction to form new compounds.
Do not stop now... There's more after the advertising ;)
Factors that regulate enzyme activity
The activity of an enzyme is influenced by factors, the main ones being temperature and the pH. Temperature generally plays a positive role in chemical reactions, increasing the rate of an enzymatic reaction. However, when the temperature increases above the optimal conditions, the reaction speed drops considerably. This is because the denaturation of proteins is observed. Most human enzymes have an optimal temperature between 35 and 40 ºC. In addition to the temperature, the pH it also influences enzymatic activity, and there is also an optimal value. For most enzymes, the optimum pH value is in the range of 6 to 8.
By Vanessa Sardinha dos Santos
Biology teacher