O benzene it is the most important aromatic hydrocarbon for Organic Chemistry. Its discovery was made in 1825 by the physicist and chemist Michael Faraday (1791-1867) in the lighting gas used in London at the time. In 1834, another scientist, the chemist Eilhardt Mitscherlich, managed to determine that his molecular formula was composed of six carbon atoms and six hydrogen atoms. (Ç6H6).
However, for a long time, chemists could not figure out what the structural formula of benzene would be. All proposed structures, as shown below, did not explain the reactions involving this compound and its chemical behavior.
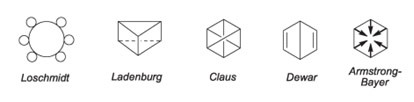
The answer to this intriguing search came in 1865, through the German chemist Friedrich August Kekulé Von Stradnitz (1829-1896). It is well known that Kekulé got help in developing the benzene structure of a dream, in which he saw a snake biting its own tail. The following text is taken from a speech he made in 1890, commemorating the 25th anniversary of the announcement of the benzene formula:
“I was sitting writing my textbook, but the work was not progressing; my thoughts were elsewhere. I turned my chair to the fire and dozed. Again the atoms were bouncing before my eyes [Kekulé had previously dreamed of atoms “bouncing” before his eyes]. At this time, the smaller groups kept modestly in the background. My mental eye, which had been sharpened by repeated visions of the same type, could now distinguish larger structures from multiple conformations: long rows, sometimes tighter, all paired together and intertwined in motion, like a snake. But look! What was that? One of the snakes had snatched its own tail, and that shape twirled mockingly in front of my eyes. I woke up as if by a ray of light; and then I also spent the rest of the night developing the consequences of the hypothesis.” (BENFEY, 1958, p. 21 apud USBERCO, SALVADOR, 2000, p. 74)
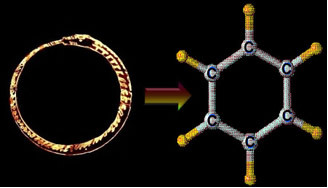
Of course, Kekulé's credit for discovering the benzene formula is not simply due to a dream, as this was just a a consequence of the days that he was diligently studying the ideas that he himself had formed of the valences of atoms and the nature of their Connections. The dream came to complete his theory of benzene. Because of this, he said:
“Let us learn, gentlemen, to dream and then perhaps we can find the truth... but let's avoid publishing them before putting dreams to the test of the real world.”(
It was even Kekulé who proposed and confirmed the tetravalence of carbon (capacity that carbon has to make four bonds).
Thus, the structural formula proposed by Kekulé for benzene was that the six carbon atoms and the six hydrogen atoms would be forming a hexagonal ring (like the snake biting its own tail). In his original hypothesis, there were only simple bonds between carbons. However, shortly thereafter, this hypothesis was improved with the addition of a pair of structures in equilibrium, with alternating double bonds.
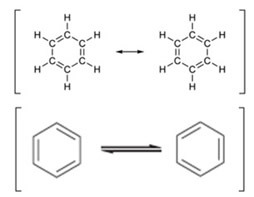
Kekulé thus anticipated the idea of the resonance of the benzene ring, which appeared only in 1930.
This discovery by Kekulé was really very important, marking history, because, through it, several other organic compounds can be synthesized.
[1] BENFEY, Journal of Chemical Education, vol. 35, 1958, p. 21. In: USBERCO, João and SALVADOR, Edgar. Chemistry 3: Organic Chemistry. Volume 3. 6th ed. São Paulo: Editora Saraiva, 2000, p. 74;
[2]
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/descoberta-estrutura-benzeno.htm
