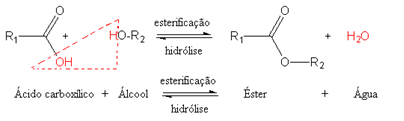
As the name implies, an esterification reaction is one in which an ester is formed. this kind of reaction it occurs between a carboxylic acid and an alcohol, also forming water, in addition to the ester.
In the case of primary alcohols, the hydroxyl group (─ OH) of the carboxylic acid joins the hydrogen in the alcohol and forms water. Generically, we have:
It is known that hydroxyl comes from carboxylic acid and not alcohol because experiments were performed in a laboratory where the oxygen in alcohol was the isotope of oxygen 18, which is a radioactive element. Thus, after carrying out the esterification reaction, as shown below, it was observed that oxygen 18 was in the ester and not in the water:
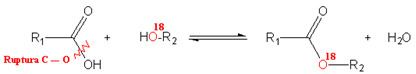
Note the example below:

Note that the esterification reactions are in dynamic equilibrium, meaning they are reversible. The reverse reaction, in which water and an ester react, is called ester hydrolysis. If it takes place in an acidic environment, the acid and alcohol will be formed exactly. But if it occurs in a basic medium, a salt of carboxylic acid and alcohol will be formed.
This reaction can also be performed between a inorganic acid and an alcohol, but the formation of water will occur in a way contrary to what was seen previously. This means that the hydroxyl will come from the alcohol and the hydrogen from the acid.
An example of this type of reaction is the one that occurs between propanediol alcohol (glycerin or glycerol) and nitric acid, with the formation of the glycerin trinitrate ester, better known as nitroglycerin, widely used as an explosive, mainly in dynamite.

This type of reaction is also very important for the food industry, as most of the flavoring (compounds produced artificially that give odor and flavor to processed foods, such as candies, cakes, ice cream, soft drinks, etc.) is an ester.
Some esters used as flavorings are:

These compounds are cheaper and easier to produce than natural additives.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/reacoes-esterificacao.htm
