sensible heat
We know that heat is Thermal energy in transit that flows between bodies due to the difference in temperature between them.
So imagine an iron bar that receives or loses a certain amount of heat (Q). This heat that the bar gained or lost is calledsensible heat, because he causes only variation in temperature of the body without changing its aggregation state, that is, if the body is solid, it remains solid.
Also called specific heat, O sensible heat, determined by the letter c (lower case), is evaluated as follows:cal/g. °C. This relationship informs the amount of heat that a gram of substance must receive or give away for the variation of one degree of temperature to occur.. This is a practical unit, that is, the one that is most used on a daily basis. However, in the International System of Units (SI), the specific heat can be given in two ways: J/kg. K or in J/kg. °C.
latent heat
Unlike sensible heat, when we supply thermal energy to a substance, its temperature does not vary, but its aggregation state changes, this is called latent heat.
This is the physical quantity that tells the amount of thermal energy (heat) that a unit of mass of a substance must lose or receive in order for it to change its physical state, that is, go from solid to liquid, from liquid to gas, and so on.
determined by the letter L, O latent heat of a substance is calculated through the ratio between the amount of heat (Q) that the substance must receive or give away and the mass (m), ie, mathematically, we have:
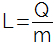
Latent heat can be positive or negative. When positive, it indicates that the material is receiving heat; when negative, it indicates that it is losing heat. In the International System of Units, the unity of latent heat is the joule per kilogram (J/Kg), but in practice, the calorie per gram (cal/g).
By Marco Aurélio da Silva
Brazil School Team
Source: Brazil School - https://brasilescola.uol.com.br/fisica/calor-sensivel-calor-latente.htm

