Radioactivity is the property that some atoms, like uranium and radio, have to issue spontaneously energy in the shape of particles and wave, becoming chemical elements more stable and lighter.
Types
Radioactivity presents itself with two ways different radiations: particle — alpha (α) and beta (β); and electromagnetic wave — gamma rays (γ).
alpha rays: they are positive particles made up of two protons and two neutrons and with low penetration power.
beta rays: are negative particles that do not contain mass consisting of an electron (negligible mass), and their penetration power is greater than that of alpha rays, but less than that of gamma rays.
Gamma: they are high energy electromagnetic waves and, as they are not particles, they also do not have mass.
Read too: Radioactivity formulas
Do not stop now... There's more after the advertising ;)
laws
The radioactive emission of particles follows certain behaviors that are explained by the laws of radioactivity (one for the alpha particle and one for the beta particle), which were described by the chemist English
Frederick Soddyand by the Polish chemist and physicist Kazimierz Fajans.First law of radioactivity
According to this law, when a radioactive atom emits alpha-type radiation, it will give rise to a new atom with core containing two protons and two neutrons less, totaling a mass four units smaller. We can represent the first law of radioactivity with the following generic equation:
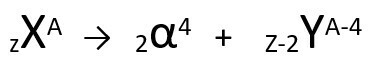
Generic equation of the first law of radioactivity.
Let's look at an example:
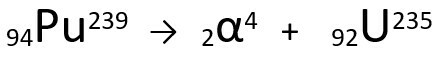
Equation representing the α-particle emission by Plutonium-239.
Note that, when emitting an alpha radiation, the newly formed atom, Uranium-235, has a mass number four units smaller and the atomic number two units smaller — exactly the values corresponding to the α particle emitted by the nucleus of the plutonium. To learn more about, go to: First Law of Radioactivity or First Soddy's Law.
Second law of radioactivity
The second law talks about the beta issue. When an atom emits a beta particle, consisting of an electron and of negligible mass, its atomic mass remains unchanged it's yours atomic number increases one unit. Generically, we represent as follows:
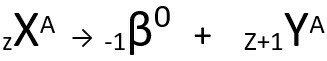
Generic equation of the second law of radioactivity.
See the example:
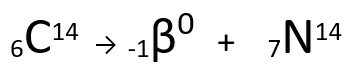
Equation representing the β-particle emission by Carbon-14.
It can be seen that the nitrogen atom formed has the same mass as the C-14 atom, that is, they are isobars, and its atomic number increases by one unit. The increase in atomic numberwas explained by the scientist Henrico Fermi, who proposed that one of the neutrons of the nucleus undergoes a transmutation, according to the following equation, generating aelectron(the emitted beta particle), a neutrino(a subatomic particle with no electrical charge and no mass, ) and a proton(P).
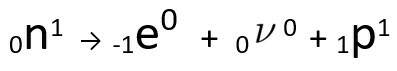
Equation representing the neutron transmutation, according to Fermi's hypothesis.
O electron it's the neutrino are issued to out of the core, remaining only the proton, which explains the increase in the atomic number To learn more about it, go to: Second Law of Radioactivity or Second Law of Soddy.
Read too: Difference between radioactive contamination and irradiation
applications
despite the negative view that deposit on radioactivity, it has important applications in our daily lives, for example, in the production of electricityin nuclear power plants through fissionof radioactive atoms.
Currently, Brazil does not use the nuclear energy as its main source of energy, but it has nuclear plants (Angra 1 and 2) working to supply electricity to the country. We can also mention the material dating found by archaeologists using carbon-14.

Rio de Janeiro nuclear power plant, Brazil
Another fundamental role that radioactivity plays is related to the area of medicine, such as in X rayand in the CT scans, and also in some types of cancer treatment.
Read too: Main risks of nuclear power generation for the environment
natural radioactivity
daily we are exposed The small quantities of radiation, whether artificial or natural. Natural radioactivity occurs spontaneously in nature. Part of this radiation that we receive comes from the food consumed on a daily basis, such as Radon-226 and Potassium-40, which are presented in very low levels and they do not place risks on our health or harm the nutritional values of foods.
This process of exposing food to radioactive emissions is intended to preserve food and promote a plant growth. Some examples of foods that emit radiation are: Brazil nuts, banana, beans, red meat, among others.
Discovery
The study of radioactivity began with research by the German physicist Wilhelm Röentgen, in 1895, when he was investigating the effect ofluminescence. Another important scientist for the development of radioactivity was the French physicist Antoine-Henri Becquerel, who noticed, in 1896, markings made on photographic film by a sample of uranium salt.
However, it was the Curie couple who used the term radioactivity for the first time. In 1898, the polish Marie Curie continued studies on radioactivity and made valuable discoveries for the area, such as the discovery of two new radioactive elements: polonium (Po) and radium (Ra).
Posteriorly, Ernest Rutherford discovered the alpha-type radiation (α) and beta (β), which allowed better explanations for its atomic model, as well as the advance of research related to radioactivity.
Read too:Marie Curie: biography, contributions and legacy
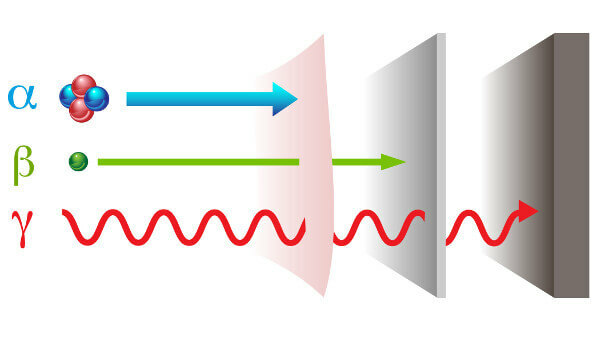
Types of radiation and their penetration powers.
decay
O radioactive decay (or transmutation) is the natural process where one unstable core emits radiation, successively, in order to lower your energy and become stable.
This normally occurs with atomic number atoms. greater than 84, which are atoms with high instability nuclear due to the amount of positive charge (protons) accumulated in the nucleus. In this process, the neutrons are not enough to stabilize all the protons clustered in the nucleus, and then the nucleus starts to undergo radioactive decay until its atomic number is less than 84.
In some cases, it may happen that atoms with an atomic number less than 84 have unstable nuclei and also go through the decay process, but for that they need to have a number of protons well above the number of neutrons.
Radioactive decay is calculated by half-life (or period of semi-disintegration, P) of the radioisotope, which is the time required for half the mass of the initial radioactive sample to disintegrate, that is, to become stable. Graphically speaking, the concept of half-life is represented below. Because it is a continuos process, the curve tends to reach zero.
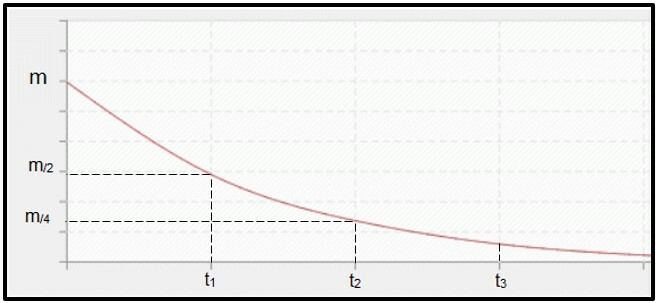
Graph representing half-life time.
Calculations involving radioactive decay follow the following formulas:
Formula for calculating the remaining mass after the half-life:
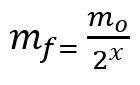
mf – final mass
mO – initial mass
x – amount of half-lives elapsed
Formula for calculating the disintegration time of a radioactive sample:
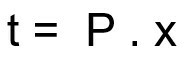
t – disintegration time
P - half-life period
x – amount of half-lives elapsed
radioactive elements
There are two types of radioactive elements: you natural and the artificial. The natural ones have elements found in nature, already with their unstable cores, such as the uranium, O actinium it's the radio. Artificial ones are produced by processes that destabilize the nucleus of an atom. In this case, we can mention the astatine it's the francium.
The main radioactive elements are: uranium-235, cobalt-60, strontium-90, radium-224 and iodine-131. Due to its wide use in nuclear power plants and cancer treatments, these elements tend to appear more frequently in our daily lives. To learn more about this subject, go to: radioactive elements.
Radioactive trash
Radioactive waste or nuclear waste it's the residue of the industries that use radioactive material in their processes that no longer have practical application. This garbage comes mainly from the nuclear power plants it's from medical applications.
The large production of radioactive waste has been a environmental problem for the whole world, due to the scarce and inadequate disposal conditions and storage.
These tailings are associated with contamination of the soil, waterways and air, resulting in destruction of the environment gradually. In addition, they also pose risks to human health, such as infections, cancer and, in more severe cases of contamination, they can lead to death.
solved exercises
(PUC-Camp-SP) The atomic bomb, also called nuclear bomb, has uranium-235 atoms as its fissile constituent, , alpha particle emitters
, alpha particle emitters 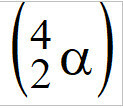 . Each atom of U-235, when emitting an alpha particle, is transformed into another element, whose atomic number is equal to
. Each atom of U-235, when emitting an alpha particle, is transformed into another element, whose atomic number is equal to
a) 231.
b) 233.
c) 234.
d) 88.
e) 90.
Template: When an atom emits an alpha particle there is a decrease of two units in the atomic number, according to the first law of radioactivity. Therefore: 92-2 = 90. Letter e.
(PUC-Camp-SP) Iodine-125, a radioactive variety of Iodine with medicinal applications, has a half-life of 60 days. How many grams of Iodine-125 will remain after six months, based on a sample containing 2.00 g of the radioisotope?
a) 1.50
b) 0.75
c) 0.66
d) 0.25
e) 0.10
Template: First, the number of half-lives elapsed during the 180 days is calculated:
t = P. x
180 = 60. x
x = 3
Once the number of half-lives elapsed is found, the mass that will remain at the end of the 180 days is calculated:
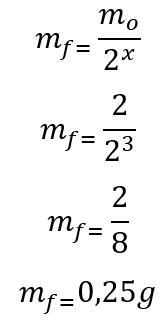
Therefore, 0.25g of the radioisotope of Iodine-135 will remain at the end of the six months. Letter D.
By Victor Felix
Graduated in Chemistry


