You organic halides are compounds resulting from the replacement of one or more hydrogens in hydrocarbon molecules by halogen atoms, which can be fluorine, chlorine, bromine or iodine.
The official nomenclature of these compounds follows the following rule below:

Organic halide naming rules.
The prefix “mono” is hardly used. Note that halogens are treated in this case as if they were a radical, that is, as an alkyl substituent.
See some examples:
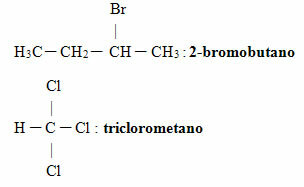
In organic halides with several branches in addition to halogen, the rule of smallest numbers is followed because, as stated, halogens are just radicals, like other alkyl substituents, so they don't have any. preference. Also, if there are unsaturation and branches, the main chain numbering starts closest to unsaturation and not halogen.
It is also necessary to number the position where the branches and unsaturations come out and place the radicals in alphabetical order, as shown below:
Do not stop now... There's more after the advertising ;)

Note, in the latter case, that the name given was not 3-chloro-5-methyl-hexane, because, as stated, we must follow the rule of smallest numbers.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Nomenclature of organic halides"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/nomenclatura-dos-haletos-organicos.htm. Accessed on June 27, 2021.



