As shown in the texts "Electromagnetic spectrum of Chemical Elements" and "Emission and Absorption Spectra and Kirchhoff's Laws”, the discontinuous emission spectra of each chemical element are different.

So, below we have the distinct spectra of some of these elements:
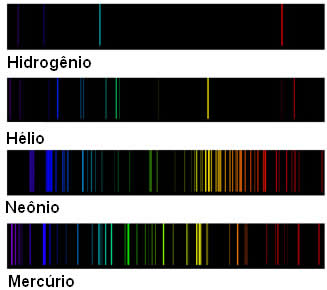
Thus, the Danish physicist Niels Böhr (1885-1962) realized that this could be related to the structure of the atom of each of these elements. So he proposed an atomic model that complemented Rutherford's model, but focused on the behavior of surrounding electrons in the atom's nucleus.
Some time earlier, Max Planck (1858-1947) had proposed a theory that electrons are quantized, in the sense that they emit and absorb specific amounts of energy, as if they were small packets of energy, which he called how much (quantum, in the singular).
Thus, Böhr proposed the following: since each element has a different spectrum, each element has in its atom electrons of constant and different energies from element to element.
Each electron can only stay in a specific specific orbit, because in each of these orbits the electron has constant, well-defined and characteristic energy. The electron can only occupy the energy levels for which it has the respective energy.
Spectra are discontinuous because electrons are quantized.
An electron can only change levels if it absorbs energy. For example, when you burn a sodium salt in a Bunsen burner, you are providing energy for the electrons. When absorbing a quantum of energy, the electron jumps to another more energetic level, staying in the excited state. However, the ground state is more stable, so this electron emits the absorbed energy and returns to its original orbit. It emits this energy in the form of electromagnetic waves that can be visualized in the form of light. In the case of sodium, this light is intense yellow in color. Thus, when these waves pass through a prism, the discontinuous spectrum of sodium is obtained.
So, for Böhr, each luminous line that appeared in the discontinuous spectrum of the elements indicated the energy released when the electron returned from one outer level to one closer to the nucleus.
The figure below helps to better understand this issue:
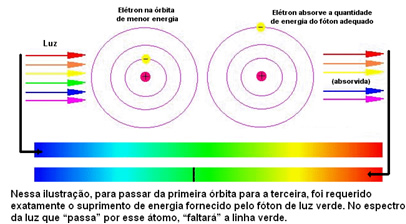
As the atoms of each element are only allowed certain energy values that correspond to the energy layers, for each element there is a different spectrum.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/espectros-eletromagneticos-estrutura-atomo.htm

