Stoichiometry is the calculation of the quantity of substances involved in a chemical reaction.This is done based on the laws of reactions and is generally carried out with the help of the corresponding chemical equations. This word, stoichiometry, is derived from the Greek: stoikheion = element, and metron = measure or measurement.
In chemical reactions, substances react with each other, originating products in specific proportions. In this way, it is possible to calculate how much product will be formed, or the reaction yield. If we want a certain yield, we can also calculate how much reagent should be used.
Through stoichiometric calculations it is possible to make these and other specific relationships. But, first of all, we need to know the proportions that exist between the elements that make up the different substances. And these proportions are given by molecular formulas, percentages and minimum or empirical.
Furthermore, the basis of the coefficients of any reaction are the weight laws:
- Mass Conservation Law– In a closed system, the total mass of reactants is equal to the total mass of the products;
- Law of constant proportions– Every substance has a constant mass proportion in its composition.
In addition Gay-Lussac volumetric law it also provides us with important information: if the pressure and temperature do not change, the volumes of gases participating in a reaction have a relationship of whole and small numbers to each other.
Do not stop now... There's more after the advertising ;)
The relationship shown below is used in stoichiometric calculations:
1 mol ↔ 6. 1023 molecules or unit formulas ↔ molar mass in g/mol ↔ 22.4 L (in CNTP*) |
*Normal Temperature and Pressure Conditions.
Let's look at an example of a stoichiometric calculation in which only amount of matter (mols) will be related.
Example:What is the amount of ethyl alcohol matter, C2H6O(1), which must react to provide 12 moles of carbon dioxide? Consider this a complete combustion reaction.
Balanced Equation:
Ç2H6O(1) + 3 O2(g) → 2CO2(g) + 3 H2O(v)
Note that 1 mole of alcohol produces 2 moles of carbon dioxide, so you can make a simple rule of three to solve the problem:
1 mol 2 mols
x12 moles
X=6 moles
Answer: 6 moles of ethyl alcohol are needed to generate 12 moles of carbon dioxide.
Remember that it is also possible to relate mass, number of molecules and molar volume. However, in all these cases it is necessary to follow the following fundamental rules:
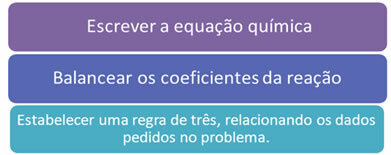
Fundamental rules of any stoichiometric calculation.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Reaction Stoichiometry"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/estequiometria-reacoes.htm. Accessed on June 27, 2021.