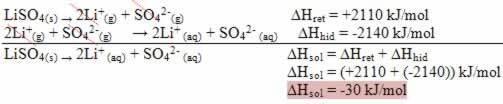
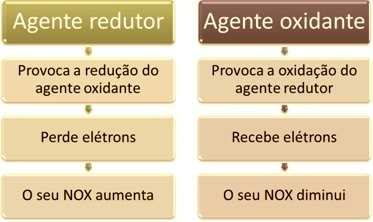
One of the main features that distinguish an oxidation-reduction reaction (or redox) of the others is the presence of an oxidizing agent and a reducing agent, which can be defined as follows:
For example, look at the chemical reaction below where aluminum (Al) corrodes in aqueous hydrochloric acid (HCl) solution. Aluminum atoms transfer electrons to H cations+(here) and produce the Al cation3+(here):
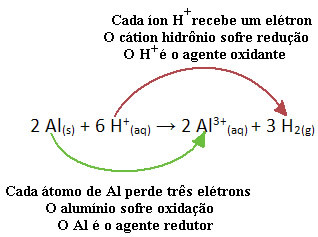
Note that since Al transferred electrons, this means that he caused the reduction of the H cations+(here); that's why he is called reducing agent. Already the cation H+(here) removed the electrons from the aluminum, causing oxidation of that metal; therefore he acts as a oxidizing agent.
In everyday life, there are many examples of the performance of oxidizing agents and reducing agents. Look at some of them and remember, however, that in all cases the reduction occurs simultaneously with the oxidation; therefore, if there is a reducing agent, there is also an oxidizing agent.
- Examples of reducing agents:
- In photographic films: photographic films contain light-sensitive silver salts. At points where there is incidence of light there is a reduction of Ag ions+, resulting in the contrast observed in the negatives.

- Vitamin C: Vitamin C (L-ascorbic acid) is a powerful reducing agent in aqueous solution. It has an exceptional facility to be oxidized and that is why it is widely used, especially in foods as antioxidant, that is, it is added to other foods and protects them from possible oxidation, due to its own sacrifice. An example is fruits such as apples and pears that darken in contact with oxygen in the air, because they oxidize. But when you add a small amount of orange or lemon juice (which contain vitamin C) to the fruit cut, this prevents this reaction from occurring, because vitamin C acts as a reducing agent and oxidizes before the fruit.

- Hydrogen gas: the hydrogen gas (H2) is used in rocket propulsion and is considered one of the most important energy alternatives, as its combustion releases a large amount of energy and no pollutants. In this reaction, hydrogen acts as a reducing agent, being oxidized by oxygen.
- Examples of oxidizing agents:
- In the production of vinegar: when wine is exposed to air, it turns into vinegar, the main component of which is acetic acid. This is because the ethyl alcohol or ethanol present in wine oxidizes on contact with atmospheric oxygen, resulting in acetic acid. Thus, oxygen is an oxidizing agent. Even the origin of the term “oxidation” is related to the reaction with oxygen.

Do not stop now... There's more after the advertising ;)
- In the rust: as stated in the previous example, oxygen acts as an oxidizing agent for alcohol; and it does this also in contact with various metals, such as iron, causing the rusting process. In addition to oxygen in the air, other oxidizing agents in this case are water or an acidic solution.
- In bleaches: the bleaching effect of the bleaches is due to the presence of the following two reducing agents: o hypochlorite anion (generally in the form of sodium salt – NaOCl), present, for example, in bleach; and hydrogen peroxide (H2O2), marketed as hydrogen peroxide. These two compounds have a very strong tendency to oxidize and cause the reduction of other chemical species. Therefore, they are responsible for oxidizing substances that impart dark color to products. For example, in cellulose, lignin is broken down and becomes lighter and more malleable. In the case of stain removal and fabric bleaching, these oxidizing agents cause the oxidation of organic molecules such as fats and dyes.

- In breathalyzers: a simple disposable breathalyzer consists of a clear tube containing an aqueous solution of the dichromate salt of potassium and silica, moistened with sulfuric acid; mixed with orange color. This salt, in contact with the alcohol vapor contained in the drunk driver's breath, reacts, changing the color to green. This means that the oxidation of ethanol (alcohol) to ethanal is caused.

By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Concept and examples of reducing agent and oxidizing agent"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/conceito-exemplos-agente-redutor-agente-oxidante.htm. Accessed on June 28, 2021.
Chemistry

Atmospheric corrosion resistant steel, Chrome, Nickel, manufactured from pig iron in blast furnaces, resistance to high temperature oxidation, Stainless steel, group of ferrous alloys resistant to oxidation and corrosion, production of parts for vehicle
Chemistry
How the Breathalyzer works, alcohol concentration, breathalyzer, reactions involving ethyl alcohol, types of breathalyzers, potassium dichromate, fuel cell, catalyst, electron release, acetic acid, hydrogen, conce
Chemistry

Photosensitive lenses, oxidation-reduction reactions, loss or gain of electrons, photosynthetic lenses in sunglasses, composition of photochromatic glass, tetrahedral oxygen atoms, crystal structure of silver chloride, ultraviolet light, silver metal