Ketones are organic compounds characterized by the presence of the carbonyl group on a secondary carbon, that is, a double bond between an oxygen atom and a carbon bonded to two other carbons, as shown. bellow:
O
║
C C ─ C
Among the best known ketones is acetone, a solvent commonly used to remove nail polish. Its structure is as follows:
O
║
H3C─C─CH3
However, the official name of this compound is not acetone. Let's find out what your name is by learning the naming rules for ketones established by IUPAC:
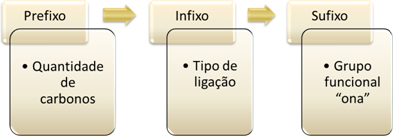
So, considering the acetone molecule, we have the following:
O
║
H3C─C─CH3→ propanone
- 3 carbon atoms = prop
- single bonds between carbons = an
- ketone functional group = one
In this case, it is not necessary to number the carbon chain because there is no other location possibility for the carbonyl. If the oxygen came attached to any of the other carbons, it would no longer be a ketone, but an aldehyde, because it would come at the end, in primary carbons.
This is also true for butanone, which will always come out of carbon 2:
O
║
H3C─C─CH2 CH3: butanone
But, in other cases, it is necessary to carry out the numbering of the chain, always starting from the end closest to the carbonyl group. If there are unsaturations or branching along the chain, it is also necessary to indicate the carbon number where they are occurring.
See the examples below:
O
║
H3Ç1 ─C2 ─C3H2 ─C4H2 ─C5H3: pentan-2-one
O
║
H3Ç1 ─C2H2 ─C3─C4H2 ─C5H3: pentan-3-one
O
║
H2Ç1 ═C2H ─C3 ─C4H2 ─C5H2 ─C6H3: hex-1-en-3-one
O
║
H3Ç5 ─C4H ─C3H2 ─C2 ─C1H3: 4-methylpentan-2-one
│
CH3
Do not stop now... There's more after the advertising ;)
CH3 O
│ ║
H3C C4H ─C3H ─C2 ─C1H3: 3,4-dimethylhexan-2-one
│
Ç5H2
│
Ç6H3
CH3 O
│ ║
H3C C6H2 ─C5H2 ─C4H ─C3H2 ─C2 ─C1H3: 4,6-dimethyl-octan-2-one
│
7CH2
│
8 CH3
There is also a common nomenclature for ketones, which is made by naming the carbonyl group (C ═ O) as a “ketone” and writing the carbon chains attached to it as being radicals. You can do this in two ways: if the word “ketone” is written after the radicals, their order will be in alphabetical order; but if the word “ketone” is written first, the order of complexity follows.
Examples:
O
║
H3C─C─CH3: dimethyl ketone or dimethyl ketone
O
║
H3C─C─CH2 CH3: ethyl methyl ketone or methyl ethyl ketone
O
║
H3C─C─CH2 CH2 CH3: methyl-propylketone or methyl and propyl ketone
O
║
H3C CH2 ─ C─CH2 CH3: diethyl ketone or diethyl ketone
O
║
H3Ç5 ─C4H ─C3H2 ─C2 ─C1H3: isobutyl methyl ketone or methyl isobutyl ketone
│
CH3

By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Ketone nomenclature"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/nomenclatura-das-cetonas.htm. Accessed on June 27, 2021.
Chemistry

Ketones, organic substances, carbonyl functional group, obtaining enamel solvent, propanone, ketone bodies in the bloodstream, extraction of oils and fats from plant seeds, solvents Organic.