Gas and Steam
The difference between gas and steam is given from the critical temperature. Vapor is matter in a gaseous state, a state that can be liquefied with increasing pressure. Gas is not the same. It is a fluid that cannot be liquefied with a simple increase in pressure. This makes gas different from steam.
Behavior of Gases
A given substance in the gaseous state is a gas if its temperature is higher than the critical temperature, if the temperature is equal to or lower than the critical temperature the substance is vapor.
The real gases that we normally know, such as helium, nitrogen and oxygen, have different and particular molecular characteristics of each one. However, if we put them all at high temperatures and low pressures, they start to show very similar behavior. In the study of gases, a simple theoretical model is adopted, which in practice does not exist, with behavior similar to that of real gases. This approximation gets better the lower the pressure and the higher the temperature. This gas model is called
Around the 17th and 19th centuries, three scientists (Jacques Charles, Louis J. Gay-Lussac and Paul E. Clayperon), after studying the behavior of gases, elaborated laws governing the behavior of perfect gases, also called ideal gases. The laws determined by them establish the rules of the “external” behavior of the perfect gas, leading to it only counts the physical quantities that are associated with them, which are: volume, temperature and pressure.
General law of perfect gases
The expression that determines the general law for perfect gases can be seen as follows:
Do not stop now... There's more after the advertising ;)
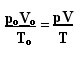
where pO, VO and TO are the initial pressure, initial volume and initial temperature, respectively. This is an expression that is used for when the variables of a gas vary.
boyle's law
Robert Boyle, physicist and chemist, was the one who determined the law that governs the transformations undergone by a gas when its temperature is kept constant. His law says that when a gas undergoes an isothermal transformation, its pressure is inversely proportional to the volume occupied. From this law we obtain that how TO = T We have to:
POVO = pV
Charles' law
Charles' law is the law that governs the transformations of a perfect gas to constant volume. These transformations are called isochoric or isometric transformations. According to this law, when a perfect gas mass undergoes an isochoric transformation, its pressure is directly proportional to its absolute temperature. Mathematically this law can be expressed as follows:

where pO and TO are the initial pressure and the initial temperature respectively.
Gay-Lussac's Law
Gay-Lussac's law is the law that governs the transformations of a perfect gas at constant pressure. This law, despite bearing the name Gay-Lussac, had already been discovered by the physicist and chemist A.C. Charles. According to the law, when a gas undergoes an isobaric transformation, the volume of the gas is directly proportional to its absolute temperature. Mathematically this law can be expressed as follows:

Where VO and TO correspond respectively to the initial volume and the initial temperature.
By Marco Aurélio da Silva
See more!!
Gas Transformations
Know what gas transformations are.
Would you like to reference this text in a school or academic work? Look:
SANTOS, Marco Aurélio da Silva. "Study of Gases"; Brazil School. Available in: https://brasilescola.uol.com.br/fisica/estudo-dos-gases.htm. Accessed on June 27, 2021.