The text "Molecule with an asymmetric carbon and optical isomers” showed that when a molecule has only one asymmetric or chiral carbon (with all the ligands different from each other), it has two optically active isomers and one optically inactive isomer (mixture racemic).
Now let's consider the case that there are two or more different asymmetric carbons, that is, they are considered different from each other because at least one of their ligands is not the same. For example, consider the formula of aspartame, which is 180 times sweeter than sucrose (sugar) and is therefore used in artificial sweeteners:
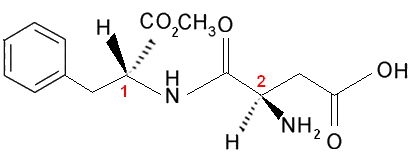
Note that this molecule has two chiral carbons, which are identified in the image by the numbers 1 and 2. These carbons have their four bonding groups different from each other, so they are considered asymmetric. When comparing carbon ligands 1 with the carbon ones 2, we see that the only repeating ligand is the H (hydrogen), being, therefore, different asymmetric carbons.
This molecule and all those that have two asymmetric carbons will always have:
4 optically active isomers and 2 optically inactive isomers (two racemic mixtures).We know this because the possible probabilities are:

Any other pairs of isomers will be diastereoisomers (which are not mirror images of each other), such as a mixture of right-handed carbon-1 and right-handed carbon-2.
However, it would be very difficult if we had to keep looking at all the possibilities for each molecule, since many have 3, 4, 5 or more asymmetric carbons.
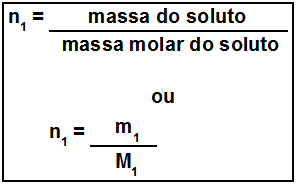
Thus, the easiest way to determine the amount of optically active and inactive isomers of a molecule that has several different asymmetric carbons is through the mathematical expressions shown below that were proposed by Van’t Hoff and Le Bel:
Do not stop now... There's more after the advertising ;)
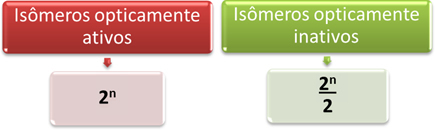
Where “n” is the amount of different asymmetric carbons in the molecule. For example, in the case of aspartame, it's two asymmetric carbons, so we have:
- Optically active isomers: 2no = 22 = 4;
- Optically inactive isomers: 2no = 22 = 2.
2 2
It gave exactly the amount we mentioned before.
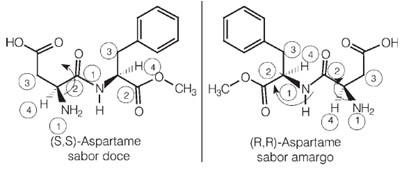
Interestingly, one of the enantiomers of aspartame has this sweet taste that is used in artificial sweeteners, but one of its optical isomers has a bitter taste.
Now look at another example, the fructose molecule:
OH O H OH OH OH
│ ║ │ │ │ │
H C ─ C ─ Ç ─ Ç ─ Ç ─ C ─ H
│ │ │ │ │
H OH H H H
Fructose has three asymmetric carbon atoms, therefore, we have 8 optically active isomers and 4 optically inactive isomers, which are 4 racemic mixtures.
- Optically active isomers: 2no = 23 = 8;
- Optically inactive isomers: 2no = 23 = 4.
2 2
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Isomers in molecules with different asymmetric carbons"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/isomeros-moleculas-com-carbonos-assimetricos-diferentes.htm. Accessed on June 28, 2021.