When we submit different elements to the action of a flame, we notice that each one emits a different color. For example, if we burn a strontium, a sodium and a copper salt, we will see, respectively, the colors red, intense yellow and green, as shown in the following figure:

If the light from these flames falls on a prism, a discontinuous spectrum, that is, only a few colored bright lines will be observed interspersed with regions without light. For each element, we will have a different spectrum.

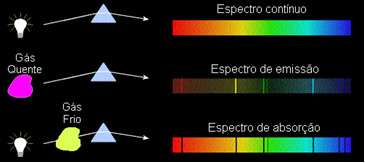
These types of spectra are called emission spectrum, as they were issued by a certain element and serve to identify it.
It is possible to obtain spectra like this by means of a light beam produced in an electric discharge tube at high temperatures and low pressures, containing gases from certain elements such as hydrogen, or as noble gases bellow:
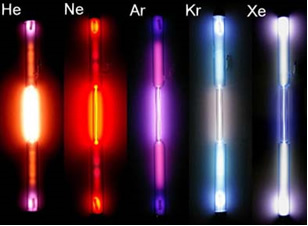
By passing this electromagnetic radiation (light) produced through a prism, the emission spectra of each of these elements are obtained.
Previously, it was thought that the solar spectrum achieved was totally continuous, but the English scientist William Hyde Wollaston found that by working with a very narrow beam of light, with a slit of about 0.01 mm, you could see that the solar spectrum contained seven black lines about it. Later, the young
Joseph Fraunhofer (1787-1826), using prisms and diffraction gratings, found that the solar spectrum actually contains thousands of superimposed black lines.Some time later the physical Gustav Robert Kirchhoff he noticed that the yellow spots, achieved by the sodium spectrum, were in exactly the same place as two black lines in the Sun's spectrum. he and the chemist Robert Wilhelm Bunsen carried out several experiments and noticed that if a white light from the Bunsen burner, such as sunlight, passed by the yellow light emitted by sodium and the prism was crossed to generate the spectrum; the result would be a continuous solar spectrum, in rainbow colors, but with the black lines (called D lines by Fraunhofer) in the same position as the yellow lines in the sodium spectrum.
Do not stop now... There's more after the advertising ;)
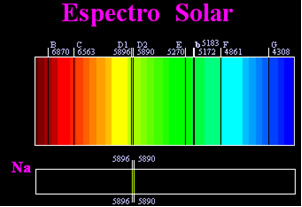
The Sun emits light of all colors, from red to violet, however, when passing through the Earth's atmosphere, the gases present absorb the Sun's light exactly in the colors they emit.
These types of spectra are called absorption spectra.
Based on these observations, Kirchoff created three laws for Spectroscopy, which are:
1) A body opaque hot, in any of the three physical states, emits a spectrum continuous.
2) A gas transparent – like those of the noble gases we saw above – produces a emission spectrum, with the appearance of lines bright. The number and position of these lines will be determined by the chemical elements present in the gas.
3) If a continuous spectrum pass through a gas at the lowest temperature, the cold gas causes the presence of dark lines, that is, a absorption spectrum. This is what happened to the spectrum of sunlight passing through the sodium gas. In this case, the number and position of lines in the absorption spectrum also depend on the chemical elements present in the gas.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Emission and Absorption Spectra and Kirchhoff's Laws"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/espectros-emissao-absorcao-leis-kirchhoff.htm. Accessed on June 27, 2021.