In our studies, we saw that thermal machine is any device that constantly converts heat into useful work, using a fluid, called a working fluid, which undergoes cyclical transformations between two temperatures that remain constants. Thus, in a thermal machine, the hot source and the cold source are systems that exchange heat without changing their temperatures, in small intervals of time.
In the same way that we come across thermal machines in our daily lives, we also come across refrigerator machines (or heat pumps). Examples: refrigerators, freezers and refrigerators. In these machines, the working fluid is subjected to a counterclockwise cycle, so it removes a certain amount of heat (Q2) from the cold source; and gives off heat (Q1) to the hot source. We know that this transfer of heat from the cold source to the hot source is not spontaneous, as it is carried out through external work. Therefore, it does not violate Clausius' statement of the second law of thermodynamics.
Do not stop now... There's more after the advertising ;)
Just to remember, the Clausius' statement says it is impossible to build a device that, operating on a thermodynamic cycle, fully converts the heat received into work.
The diagram shown in the figure below schematically represents a refrigeration machine in which work is converted into heat.

A domestic refrigerator, for example, is a refrigerating machine in which the cold source is the freezer, the hot source is the environment, and the work is carried out by the compressor.
THE efficiency (e) of a refrigeration machine is the relationship between the amount of heat removed from the cold source (Q2) and the external work (T) required for this transfer. Then:
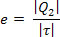
By Domitiano Marques
Graduated in Physics
Would you like to reference this text in a school or academic work? Look:
SILVA, Domitiano Correa Marques da. "Refrigerator machines"; Brazil School. Available in: https://brasilescola.uol.com.br/fisica/maquinas-frigorificas.htm. Accessed on June 27, 2021.


