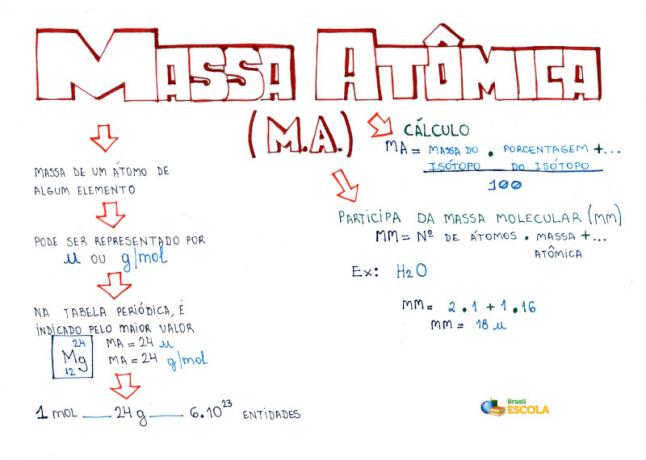
O calculation of atomic mass of an element is the mathematical resource used to determine the mass value present in the Periodic Table of each of the existing chemical elements. In general, to carry out the atomic mass calculation, we must know the following variables of the chemical element:
Element isotopes;
Mass number of each isotope of the element;
Percentage of each isotope of the element in nature.
The calculation of the atomic mass of Hydrogen, for example, was performed based on the following data:
Presents the protium isotopes (H1), deuterium (H2) and tritium (H3);
The masses of these isotopes are 1, 2 and 3 respectively;
The percentage of Protium in nature is 98.9%;
The percentage of Deuterium in nature is 0.09%;
The percentage of Tritium in nature is 0.01%.
Do not stop now... There's more after the advertising ;)
Mathematical standard for calculating atomic mass
To carry out the atomic mass calculation of any chemical element, we must use the following mathematical pattern:
1O: multiply the mass number of each isotope by its percentage;
2O: add all the results found in the first step multiplications;
3O: divide the found sum by 100.
M.A. = mass number.percentage + mass number.percentage
100
Mind Map: Atomic Mass
*To download the mind map in PDF, Click here!
Examples of Atomic Mass Calculation
⇒ 1st example: Calculation of the atomic mass of Sulfur.
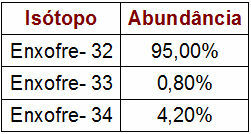
Percentage and Mass Data of Sulfur Isotopes
The table provided indicates the mass number and percentage of each sulfur isotope in nature. To perform the atomic mass calculation, simply perform the following steps:
1O Step: multiply the mass number of each isotope by the value of its abundance.
Sulfur - 32 (S32)
s32 = 32.95
s32 = 3040
Sulfur - 33 (S33)
s33 = 33.0,8
s33 = 26,4
Sulfur - 34 (S34)
s34 = 34.4,2
s34 = 142,8
2O Step: add the values found in the multiplications of the mass number and the abundance of the isotope.
Sum = S32 + S33 + S34
Sum = 3040 + 26.4 + 142.8
Sum = 3209.2
3O Step: calculate the atomic mass by dividing the sum of the results found by 100:
M.A. = Sum
100
M.A. = 3209,2
100
M.A. = 32,092 u
⇒ 2nd example: A given chemical element (D) has three isotopes, whose mass numbers are:
30D 32D 34D
Knowing that the atomic mass of this element is 32.20 u and that there is 20% of the isotope 32D in nature, what is the value of the percentages of the other isotopes?
The statement gives the mass numbers, the atomic mass and the percentage of one of the isotopes. To determine the percentages of other isotopes, we must perform the following steps:
1O Step: determine the percentage of each isotope.
Isotope - 30 (DS30)
DS30 = 30.x
DS30 = 30x
Isotope - 32 (DS32)
DS32 = 32.20
DS32 = 640
Isotope - 34 (D34)
DS34 = 34.y
DS34 = 34y
2O Step: use all the data found in the mathematical expression to calculate the atomic mass.
M.A. = mass number.percentage + mass number.percentage
100
32,2 = 30x + 640 + 34y
100
32,2.100 = 30x + 640 + 34y
100
3220 - 640 = 30x+34y
30x + 34y = 2580
x = 2580 - 34y
30
3O Step: use the expression found above from the following reasoning:
Percentage of Isotope 1 + Percentage of Isotope 2 + Percentage of Isotope 3 = 100%
x + 20 + y = 100
x + y = 100 – 20
x + y = 80
x = 80 - y
2580 - 34y = 80-y
30
2580 – 34y = 30. (80-y)
2580- 34 y = 2400 - 30y
2580 – 2400 = 34y-30y
4y = 180
y = 80
4
y = 45%
4O Step: calculate the percentage value of x in the expression constructed in the third step.
x + y = 80
x + 45 = 80
x = 80 - 45
x = 35%
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "Atomic mass calculation"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/calculo-massa-atomica.htm. Accessed on June 27, 2021.