For a chemical reaction to occur it is necessary to satisfy four basic conditions, which are:
1. Reagents must contact;
2. There must be chemical affinity between the reagents;
3. Collisions between reagent particles must be effective;
4. Activation energy must be reached.
Briefly see each case:
1.Contact between reagents:
This condition is obvious, because even if the reactants have a lot of affinity with each other, as in the case of acids and bases, if they are separated, the reaction will not occur. They need to make contact so that their particles can collide, breaking the bonds of the reactants and forming the bonds of the products.

2.Chemical affinity:
As we have seen, bringing the reagents into contact is necessary, but not enough. For example, if we put sodium in contact with water, an extremely violent reaction will occur, but if we put gold, we won't see any difference. This is because different substances have different chemical affinities with each other, or they may not have any affinity at all. The greater the chemical affinity, the faster the reaction.
In the examples mentioned, sodium has a great affinity with water, so much so that in order not to come into contact with the moisture in the air, metallic sodium is stored in kerosene. Gold is inert, which is why gold monuments last so long, like the sarcophagi of Egypt.

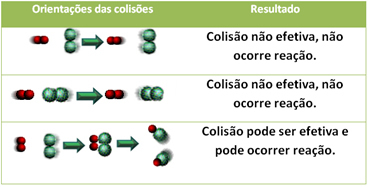
3.Collision theory:
Even in compounds that have chemical affinity, for the reaction to take place it is necessary that their particles, atoms or molecules collide effectively. Not all particles that collide do this effectively, but shocks that result in breaking the reagent bonds and formation of new bonds are those that occur in the correct orientation and with the energy enough.
Do not stop now... There's more after the advertising ;)
Shown below is the case of two ineffective collisions and one effective collision that results in the reaction occurring.
4.Activation energy and activated complex:
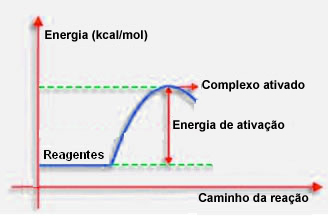
As stated in the previous item, effective collision, in addition to favorable guidance, also needs sufficient energy. The minimum amount of energy needed for each reaction to take place is called the activation energy.
If the reactants have an energy equal to or greater than the activation energy, during the well-oriented shock, an initially activated complex will form, which is an intermediate structure between the reactants and the products. In the activated complex, there are weakened reagent bonds and new product bonds forming.
Thus, the activation energy works as a kind of barrier for the reaction to occur, because the bigger it is, the more difficult it will be for the reaction to occur. In some cases, it is necessary to supply energy to the reagents. For example, cooking gas has an affinity to interact with oxygen in the air, but we need to supply energy when we bring the match together, otherwise the reaction doesn't take place. But once started, the reaction itself releases enough energy to activate the other molecules and keep the reaction going.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Conditions for the Occurrence of Chemical Reactions"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/condicoes-para-ocorrencia-reacoes-quimicas.htm. Accessed on June 27, 2021.


