question 1
Suppose two moles of a certain gas undergo a transformation as shown in the graph below the pressure vs. temperature. The universal gas constant R = 8.31 J/mol. K, molar heat at constant volume Cv = 4 cal/mol. K and the mechanical equivalent 1 cal = 4.18 J, determine the change in internal energy and mark the correct alternative.
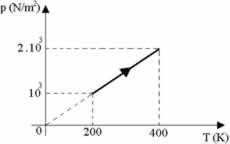
a) 8,866 J
b) 4,433 J
c) 6,975 J
d) 3,500 J
e) 6,688 J
question 2
A given gaseous mass undergoes an isothermal transformation, as shown in the diagram, and receives 2000 J from the external environment in the form of heat. Given the universal gas constant R = 8.31 J/mol. K, respectively determine the Final Volume, a internal energy variation it's the work done by gas and mark the correct alternative.
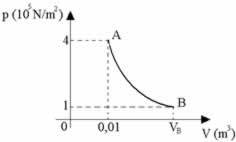
a) 0.04 m3, 200 J, 100 J
b) 0.04 m3, 10J, 5J
c) 0.04 m3, 0 J, 3200 J
d) 0.04 m3, 0 J, 2000 J
e) 0.04 m3, 200 J, 200 J
Watch our video class about the UN - United Nations, and understand what this international organization is. Learn about the UN's history and its importance to the world.
In this video lesson, you will learn a little more about the life of Edgar Allan Poe, one of the writers most important of the 19th century, which left an important legacy for the 20th century and the contemporaneity. Check out our review!