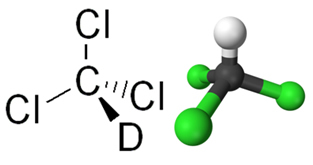
O chloroform is a chemical compound from the group of organic halides, where this group is characterized by the replacement of one or more hydrocarbon hydrogens by halogens (chlorine, fluorine, bromine or iodine). Chloroform is actually the trichloromethane(CHCl3), in which three hydrogens of methane were replaced by chlorine atoms, as can be seen in its structural formula below:
The first obtainment of chloroform was in 1831, by Liebig and Souberian, through the reaction of ethanol with chlorine gas and hot dilute sodium hydroxide solution:
oh O
│ ║
H3C─ CH2 + Cl ─ Cl → H3C─ CH + 2 HCl
The Cl O
║ │ ║
H3C─ C ─ H + 3 Cl ─ Cl → Cl ─ C ─ C ─ H + 3 HCl
│
Cl
ClO Cl O
│ ║ │ ║
Cl ─ C ─ C ─ H + NaOH →Cl─C─H + NaO ─ C ─ H
│ │
Cl Cl
Chloroform
Currently, the commercial method for obtaining chloroform is in another way, it occurs through the reduction of carbon tetrachloride (CCl4):
Do not stop now... There's more after the advertising ;)
Cl
|
CCl4 + 2 [H] → Cl─C─H + HCl
│
Cl
Chloroform
In early 19th century England, chloroform was inhaled at parties. Each guest carried a bottle and snorted it until it dropped. Nowadays, inhalant drugs known as
"lolo" or "perfume launcher"contain chloroform, which causes the person who inhaled it to become euphoric and aggressive, in addition to causing mental confusion, pallor, blurred vision, loss of self-control, hallucinations, seizures, unconsciousness, coma and death.Since its euphoric effect quickly wears off, the person starts to inhale more and more amounts of this substance, leading to dependence. Chloroform causes irritation to the skin, eyes and respiratory tract. If ingested, it causes burns, chest pain and vomiting, which can lead to the development of cancer and death. Furthermore, it destroys neurons and causes irreversible damage to the brain.

At "chloroform parties" in England, guests snorted this substance until it dropped.
However, the main use of chloroform was as anesthetic in surgeries through its inhalation. The first to use it for this purpose was the English Court doctor, sir James Young Simpson (1811-1870), in 1847. He used chloroform in obstetrics to reduce pain caused by childbirth and in general surgery.
This represented an advance for medicine, as it allowed the surgeon to do his work with more time and reduced the patient's shock.
However, over time, it was observed that the use of chloroform as an anesthetic brought several adverse effects, as the fact that it oxidizes easily in the presence of light, producing carbonyl chloride (phosgene), which is a toxic gas, which has already caused several fatal accidents. Chloroform can cause hepatic and renal necrosis in the patient and also chloroform syncope, which is a sudden cardiac arrest, early in the administration of this anesthetic.
Due to these high risk factors for the patient and with the discovery of new, safer anesthetics, the use of chloroform as an anesthetic was abandoned. Today its use is restricted to organic solvents.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Composition and Applications of Chloroform"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/composicao-aplicacoes-cloroformio.htm. Accessed on June 28, 2021.
Chemistry

Dichloro-Diphenyl-Trichloroethane, Low Cost Insecticides, World War II, Malaria, Typhus, Yellow Fever, Cumulative Effect on the Body, Agricultural Pests, Neurological Damage.
Chemistry

Tear gas, violent protests, World War I, Halide class, group of Halogens, incessant tears, o-Chlorobenzylidene malononitrile, incapacitating agent, sensation of burn.