O Acetic Acid is a colorless liquid with an irritating, penetrating odor and sour taste, which is chemically called ethanoic acid and its structural formula is shown below:
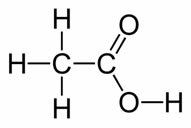
See that it is a compound that belongs to the organic group of carboxylic acids.
He is the main constituent of the vinegar, which is an aqueous solution of 4 to 10% by mass of acetic acid. It was first obtained using ethanol from wine, which oxidizes with oxygen in the air. Hence the origin of its name, as sour wine comes from Latin acetum which means “vinegar”.
The use of this compound is very old, including the Roman legions that conquered a large part of the world, known in the 3rd century BC. C., marched long distances and carried with them a flask containing diluted sour wine. This mixture of acetic acid stimulated the soldiers' salivation and lessened the sensation of thirst.

Nowadays, the industry usually uses the same principle, that is, the oxidation of ethanol (ethyl alcohol), to produce this acid:
Do not stop now... There's more after the advertising ;)
H3C CH2 ─ OH+O2 (air) → H3C COOH + H2O
Ethanol oxygen acetic or ethanoic acid Water
In the case of vinegar, this oxidation shown in the chemical reaction above is obtained through fermentation, with the help of the fungus Mycoderma aceti (called vinegar mother) and the alcohol oxidase enzyme. You can also use bacteria of the genus Acetobacter and Clostridium acetobtylicum. However, another means is the use of a catalyst such as divanadium pentoxide (V2O5).
Acetic acid can also be produced by oxidizing methanol, distilling wood and using petroleum derivatives.
In addition to being used as a seasoning in food, acetic acid is also used in the production of vinyl acetate (to make the PVA polymer), acetic anhydride and acetyl chloride (used in organic syntheses), of esters (solvents, perfumes, essences, among others), of cellulose acetate (textile fibers), of acetates inorganics etc.
When it is in its pure form it is called glacial acetic acid, as it solidifies at a temperature of 16.7ºC, giving it the appearance of ice.

By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Acetic Acid"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/Acido-acetico.htm. Accessed on June 28, 2021.
Degree of Ionization, Hydrochloric Acid, Volatility, Acetic Acid, Svante Arrehenius, Acids Conduct Electricity, neutralization reactions, Reaction with carbonates and bicarbonates, red phenolphthalein solution, litmus paper blue.
Chemistry

Carboxyl functional group, carboxylic acids, ethanoic acid, acetic acid, vinegar, methanoic acid, formic acid, benzoic acid, fungicide, low molecular weight carboxylic acids.