In the text "Concentration in mol/L or Molarity” you saw that the concentration in mol/L relates to the amount of matter in the solute (n1, measured in mols) with the volume of the solution in liters (V). However, there are some solutes that, when placed in water, generate ions.
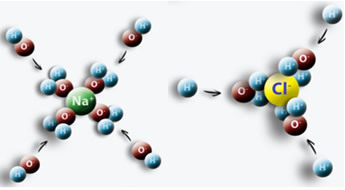
An example is when we put salt (NaCl) in water, an attraction occurs between the positive pole of the water with Cl and the negative pole with Na, giving rise to Na ions+ and Cl-.
It is important to know how to determine the concentration in mol/L of these ions, as this can be useful in many situations. One area that uses this is medicine, especially in medical blood tests. Our blood and other bodily fluids have ions dissolved in them. If the concentration of these ions in our blood is above or below normal, it can compromise the proper functioning of our body and lead to disorders and diseases.
See the case of iron ions (Fe2+): if the person lacks this ion, he may develop anemia. Thus, when someone who has anemia is told that they need to consume iron-rich foods or even some medicine containing iron, it is actually the iron ion and not the metallic element.
Another case is when there is loss of calcium ions (Ca2+) in bones, causing osteoporosis, as shown in the photos with enlarged images of bones below:

Look at other examples of ions that have vital functions in our body and need to be in the correct concentration:

Therefore, given this information, it becomes evident that knowing how to calculate the concentration of ions in solutions is really important. But how can this be done?
It is possible to determine the concentrations in mol/L of the ions present in solutions if two essential data are known, which are:
- The formulas of substances dissolved in water;
- The molarity of your solutions.
With these data in hand, write the dissociation or ionization equation of the solute in question and find by deduction the concentration of the ions, as it is proportional to the number of moles of each ion. This means that the concentration of ions is proportional to their respective coefficients in the ionization or dissociation equation.
Do not stop now... There's more after the advertising ;)
See the following example to better understand how this calculation is performed:
"Consider that the concentration in quantity of matter of a given aqueous solution of magnesium phosphate (Mg3(DUST4)2 is 0.5 mol/L. What will be the concentration in mol/L of magnesium cations (Mg2+(here)) and phosphate anions (PO3-4(aq)) in this solution, considering that the degree of dissociation (α) was 100%?”
The first step in solving this problem is to write the dissociation or ionization equation. If you have difficulty, you can base yourself on the generic equation below:
1 AxBy → x A+y + y B-x
1 mol x mol y mol
So, considering in this example that magnesium phosphate completely dissociated, we have the following equation:
1 mg3(DUST4)2(aq) → 3 mg2+(here) + 2 gp3-4(aq)
1 mol 3 mol 2 mol
We can see that 1 mole of Mg3(DUST4)2 gives 3 mol of Mg2+(here) and 2 mol of PO3-4(aq); thus, the number of moles of the cation will be 3 times the number of moles of the phosphate with which the solution was prepared, and that of the anions will be 2 times.
As the solution is 0.5 mol/L of Mg3(DUST4)2(aq) , this means that 0.5 mol of Mg was dissolved in 1 L of it3(DUST4)2, which gave rise to 1.5 mol/L of Mg2+(here) and 1.0 mol/L of PO3-4(aq):
1 mg3(DUST4)2(aq) → 3 mg2+(here) + 2 gp3-4(aq)
1 mol 3 mol 2 mol
0.5 mol x y
1. 0,5 3. 0,5 2. 0,5
0.5 mol/L 1.5 mol/L1.0 mol/L
But what if the degree of dissociation or ionization was not 100%? Let's say it was 70%, how would we go about finding out the concentration in mol/L of these ions?
In that case, just perform a simple rule of three. See below:
1.5 mol/L of Mg2+(here) 100 %
x 70%
x = 70. 1,5 → x = 1.05 mol/L of Mg ions2+(here)
100
1.5 mol/L of PO3-4(aq) 100 %
y 70%
y = 70. 1,0 → y = 0.70 mol/L of PO ions3-4(aq)
100
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Ion concentration in mol/L"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/concentracao-dos-ions-mol-l.htm. Accessed on June 28, 2021.
Chemistry

Dissociation and Ionization, Italian Scientist Volta, Electric Current, Swedish Physical Chemist Svant August Arrhenius, Theory of Arrhenius, positive ions, cations, negative ions, anions, caustic soda, table salt, polar molecules, dissociation ionic,