To understand why when we burn wood, it doesn't melt, let's first remember what a molten substance is, that is, it is in a liquid state.
The difference between solid, liquid and gaseous states is only in the aggregation state of the particles. In the three states, substance is composed of the same particles, but there is more or less space between them.
In the solid state, particles are closer together, without much freedom of movement. In the liquid state, particles have more freedom and can move.
It turns out that much of the wood (50%) is made from cellulose, which is actually a polymer. A polymer is formed by the union of several molecules, called monomers. In the case of cellulose, it is formed by the union of hundreds of β-glucose molecules, as shown below. Cellulose then has the formula (C6H10O5)no and reaches molecular masses on the order of 400,000 u.
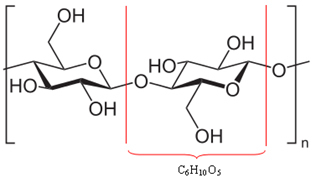
Since they are huge molecules, these polymers that make up wood cannot move easily. Also, note that the β-glucose molecules that make up cellulose have hydroxyl groups (─ OH). These groups bond hydrogen to each other, which is the most intense type of intermolecular force, keeping the polymer tightly together.
Do not stop now... There's more after the advertising ;)

Hydrogen bonds between molecules that make up the cellulose polymer*
Thus, to break these bonds, we would have to put so much energy into the system that the wood decomposes even before it melts and then is no longer wood.
Thus, when burned, wood undergoes a chemical reaction, which is more than a simple change in physical state. Its molecules end up breaking down and recombining with the oxygen present in the air, then forming new substances, such as carbon gas and water.

* Source and author of the image: laghi.l.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Why doesn't wood melt?"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/por-que-madeira-nao-derrete.htm. Accessed on June 28, 2021.
Chemistry

Main sources of carbohydrates, natural sources of carbohydrates, role of carbohydrates in the body, carbohydrates, energy action, group of macronutrients that constitute the main source of energy, disease prevention, horm changes
Chemistry

Wood, woody fabric from trees, cellulose pulp, rayon, tar, tannin, cellulose acetate, leather tanning, cellulose trinitrate, cotton powder, manufacturing of explosives.