As stated in the text "Alpha emission (α)”, New Zealand chemist Ernest Rutherford performed an experiment in which he placed a sample of a radioactive material in a lead block, with a hole to direct the radioactive emissions; and subjected these radiations to an electromagnetic field.
Among the results obtained, Rutherford noticed that a beam of radiation was attracted by the positive plate, which led him to conclude that these emissions were of negative charge. This radiation was called raysor beta emissions (β).
Since rays suffered deflection when subjected to an electromagnetic field, this also led him to conclude that they were actually composed of particles that have mass. The mass of these particles, however, was smaller than that of the particles that constituted the alpha emissions, because the β particles suffered greater deviation.
- Constitution:
In 1900, the French physicist Antoine-Henri Bequerel (1852-1908) compared these deviations suffered by the beta particles with the shifts that electrons performed when they were also subjected to a field electromagnetic. The result was that they were the same; with that, it was seen that
beta particles were actually electrons.As a result, the representation of this particle is given by 0-1β or β-. Note that the beta emission has a mass number (A) equal to zero, as the electrons are not part of the atom's nucleus.
- Consequences of beta particle emission for the structure of the atom:
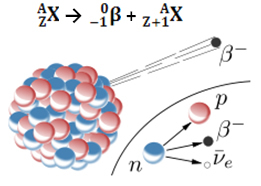
The emission of a beta particle (0-1β) is the result of rearrangement of the unstable nucleus of the radioactive atom in order to acquire stability. Therefore, a phenomenon occurs in the nucleus, in which a neutron decomposes, originating three new particles: a proton, an electron (particle β) and a neutrino. The antineutrino and electron are emitted; the proton, however, remains in the nucleus.
10no →11p + 0-1and + 00ν
neutron proton electron neutrino
Thus, when an atom emits a beta particle, it changes into a new element with the same mass number (because the neutron that existed before has been “replaced” by the proton), but its atomic number (Z = protons in the nucleus) increases by a unity.
See below how this happens in a generic way:
Here is an example of beta decay that occurs with isotope 14 of the element carbon:

Beta radiation consists of electrons emitted at high speed by the nuclei of radioactive atoms, this initial speed is from 100 000 km/s up to 290 000 km/s and reaches 95% of the speed of the light.
The mass of β radiation is the same as that of an electron, which is 1840 times smaller than that of a proton or neutron. Alpha (α) radiation emits two protons and two neutrons, so the mass of α particles is 7360 times that of β particles. This explains the fact that α particles suffer a smaller deviation than β particles, as Rutherford had verified in his experiment.
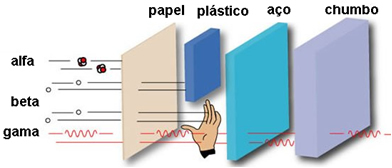
- Penetration power:
Its penetration power is medium, being 50 to 100 times more penetrating than alpha particles. These can pass through a sheet of paper, but are held by a sheet of only 2 mm lead or 2 cm aluminum. When they affect the human body, they can penetrate up to 2 cm.
- Damage to humans:
Since its penetration power over the human body is only 2 cm, the β particles can penetrate the skin, causing burns, but are stopped before reaching Organs most internal organs of the body.
By Jennifer Fogaça
Graduated in Chemistry