Living matter consists of inorganic molecules, per organic molecules and by ions.
The classification between inorganic and organic substances occurred in the middle of the 18th century and it was said that the first group consisted of compounds of mineral origin, without life. The group of organic compounds, on the other hand, originated from living plant and animal organisms. However, this concept became incorrect over time, as some organic substances were also synthesized in the laboratory.
Thus, the inorganic molecules became those that do not contain the element carbon (with some exceptions, such as carbon dioxide (CO2)).
Already organic molecules are those in which the main constituent is the element carbon, which is mainly associated with the atoms of hydrogen (H) and also to sulfur (S), phosphorus (P), nitrogen (N) and oxygen (O).
THE water (H2O) is inorganic molecule very important and is the most abundant because it corresponds to almost half of the entire constitution of living matter in nature.
It makes up more than 70% of our body and is present in numerous reactions that originate and sustain life. For example, the fertilization process is facilitated by hydrolysis, which is the chemical reaction in which a bond is broken through the addition of a water molecule. Without water there would also be no transport of oxygen from the blood and nutrients to the cells.
Water is also present in the maintenance of plants, as without it there would not be the process of photosynthesis which carries out plant nutrition.

In the water there are several ions, which are atoms or groups of atoms that have a positive electronic charge (cations) or a negative charge (anions). These ions are present in smaller amounts in living beings, corresponding to approximately 1% approximately, but they are also essential for the maintenance of life.
Do not stop now... There's more after the advertising ;)
For example, the sodium cation (At+), which can be ingested through salt (NaCl), fish and meat, is responsible for regulating body fluids. Lack of this ion can cause anxiety, diarrhea and circulatory problems. Of course, its excess is also bad, and can lead to urinary retention, thirst and edema.
Calcium (Here2+) makes up 90% of our bones and teeth and is responsible for strengthening the bone structure, as well as acting as a muscle relaxant. Its deficiency leads to tetanus, muscle cramps and osteoporosis, its excess leads to muscle relaxation, bone pain and kidney stones.
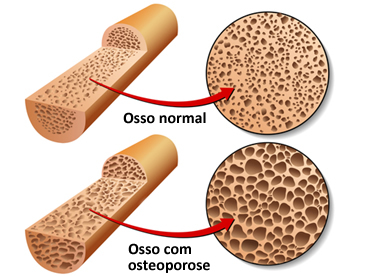
These are just two of several examples of ions that are part of the chemical makeup of living matter.
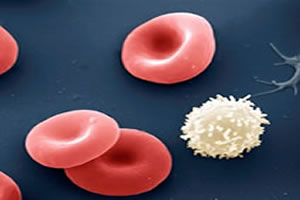
But, the molecules that are most present in living organisms are organic molecules. For example, our body is made up of over 60% organic compounds by mass. At red and white blood cell membranes of our blood are made up of lipids and proteins, which are organic molecules. The DNA itself, which transmits the hereditary characteristics of each species, is basically made up of proteins.

THE glucose produced in photosynthesis is formed only by carbons, hydrogens and oxygens (C6H12O6), that is, it is an organic molecule.
O breast milk it is rich in water and various organic chemical compounds such as carbohydrates, proteins, fats and vitamins.
Fruits and vegetables, seeds and grains, dairy products and meat are foods consisting of one or more nutrients, such as vitamins, carbohydrates, lipids (fats), proteins, mineral salts and water, which we have to ingest for our body to continue functioning regularly.
The importance of organic molecules for living matter is shown through the carbon cycle, in which the flow of energy and organic matter maintains the biological balance and recycles the element carbon.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Chemical constitution of living matter"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/constituicao-quimica-materia-viva.htm. Accessed on June 27, 2021.
Chemistry
Classification of matter, water, hydrocyanic gas, carbon dioxide, ammonia, hydrogen, helium, substances simple, compound substances, mixtures, phases of a mixture, homogeneous mixture, mixture heterogeneous.
Chemistry

Matter, Dalton's atomic theory, substances, chemical element, John Dalton, atom, body, object, constitution of matter, rearrangement of atoms, sphere, mass.

