Want to understand the differences between conductive and insulating materials? So this text is for you. Check out!
Conductors are materials that enable the movement of electrical charges inside it with great ease. These materials have a large amount of electrons free, which can be conducted when we apply a potential difference to them. Metals like copper, platinum and gold are good conductors.
The materials insulators are those that offer great opposition to the passage of electrical charges. In these materials, electrons are, in general, strongly bound to atomic nuclei and, therefore, are not easily conducted. Materials such as rubber, silicone, glass and ceramic are good examples of insulators.
Conductivity x Resistivity
The physical property that indicates whether a material is a conductor or an insulator is its resistivity, also known as specific resistance. The resistivity, whose symbol is the ρ, is measured in Ω.m, according to the International System of Units. In addition to resistivity, there is greatness
conductivity, denoted by the symbol σ, the conductivity of a material is the inverse of its resistivity, that is: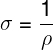
Conductivity and resistivity are inversely proportional quantities.
Conductivity and resistivity are inversely proportional quantities, that is, if a material has a high resistivity, its conductivity is low and vice versa. Likewise, given the same conditions, a conductive material does not have characteristics of insulating materials. The unit of measure of conductivity is Ω-1.m-1.
According to classical physics, the resistivity of a material can be calculated using microscopic and more fundamental quantities, such as the charge and the pasta of electrons, in addition to two quantities of great importance for the study of electrical properties of materials: o medium free path it's the average free time. Such explanations come from a physical model for driving known as drude model.
The mean free path of electrons refers to the distance they can be carried inside a material without colliding with the atoms that make up the crystal structure of the material, while the average free time is the time interval that the electrons can travel the free path average. In conductive materials, both mean free path and mean free time are significantly longer than in insulating materials, in which electrons cannot move easily.
Do not stop now... There's more after the advertising ;)
See too: electrical charges in motion
According to Drude's model, electrons move (vibrate and translate) inside conducting materials, due to their temperature, but also due to the application of an electrical potential. The speed at which electrons move, however, is extremely high, unlike yours. driving speed, which is of the order of few centimeters per hour. This happens because, despite moving at high speeds, electrons suffer constant collisions with the atoms that make up the material, thus losing part of their speed.
The resulting movement of these collisions is not null, as the electrons drag in the direction of the electric current, however it is very slow. In insulating materials, on the other hand, the mean free path of the electrons is so small that, unless a very large potential difference is applied, no electrical current is formed.
Why are some materials insulating and others conductive?
Currently, the explanation for the electrical current conduction capacity of materials is based on complex theoretical arguments that involve quantum aspects of matter. The theory behind this explanation is called theoryinbands.
According to band theory, in insulating materials, electrons have energy levels below the minimum necessary to be conducted. In conductive materials, on the other hand, electrons have energy levels greater than the minimum energy for their conduction to occur.
An amount of energy separates the electrons that can be conducted from those that cannot. This energy is called gap. In insulating materials, the gap it is very large and therefore it is necessary to apply a large amount of energy to it so that its electrons move from one point to another. As for conductive materials, the gap of energy is zero or very small, so electrons can easily move around inside it.

In materials like rubber, the gap energy is very high
Conductive materials
Conductive materials share a common characteristic: electrical current is easily conducted through them. Its main features are the abundance of free electrons, in addition to low electrical resistances.
When electrical materials are electrically charged, without carrying charges, we say that they are in balanceelectrostatic. In this condition, the electrons occupy the outermost layers of the material, positioning themselves exclusively on its surface, due to the repulsion between their charges and their great mobility.
See too: Coulomb's Law
→ Example of electrical conductors
In general, metals are good electrical conductors and, therefore, they are widely used in the transmission of electrical current, in electrical circuits and in electronic devices. In addition to metals, some salts, when dissolved in liquid media, also allow the formation of electrical currents. Check out some examples of conductive materials:
Copper
Aluminum
Gold
Silver

Aluminum is an example of an electrically conductive material.
Insulating materials
You insulating materials they offer resistance to the passage of electric current and, therefore, are widely used to block its passage. When electrically charged, these materials “trap” the charges within them. Some insulating materials can be polarized, that is, when exposed to a strong electric field external, form in its interior a contrary electric field, making the formation of electric currents even more difficult. The insulating materials capable of exhibiting such behavior are called dielectrics and are widely used in capacitors, for example.
See too:Electric field
→ Examples of insulators
Insulators strongly oppose the movement of loads and are therefore used to insulate surfaces of contact, avoiding accidents with electric shocks or reducing energy losses in conductor wires. Check out some examples of insulating materials:
Rubber
Plastic
Glass
Ceramics

Copper wires, used in motors and circuits, receive a layer of insulating varnish.
Can an insulator become a conductor?
Under special conditions, such as high temperatures, mechanical stress or huge potential differences, insulating materials become conductive. When this happens, the electrical current that passes through them usually causes a great deal of heating due to of the Joule effect, that is, due to the collisions between the electrons and the atoms that constitute the material in question.
The simplest example of breakdown of dielectric strength is that of the formation of rays: the electric field that forms between the charged clouds and the ground is so big that the air becomes ionized, allowing electrons to bounce from atom to atom. However, even being able to conduct electrical current, air becomes an insulating medium again after atmospheric discharge.
See too:What is electrostatic shielding?
Summary on conductors and insulators
Conductive materials, such as silver and copper, offer little resistance to the passage of electrical current;
Conductive materials have a large number of “free” electrons, loosely bound to atomic nuclei, called conduction electrons;
Insulating materials such as glass, rubber or ceramic offer great resistance to the passage of electrical current;
Insulating materials have a reduced number of electrons and most of them are tightly bound to their nuclei.
By Me. Rafael Helerbrock

