What is Thermology?
Thermology is the scientific study of phenomena related to heat and the temperature, such as heat transfer, thermal equilibrium, transformations undergone by gases, changes in physical state, etc.
Temperature
Temperature it is the measure of the degree of agitation of the particles that make up a body. The temperature of a body is directly proportional the speed at which its atoms and molecules vibrate, rotate or even translate.
The temperature is one of the greatnessesfundamentals of nature, together with the subway It is like second, for example. At the systemInternationalinunits (SI), the unit used to measure temperature is Kelvin (K). This temperature scale is considered absolute, as it does not admit negative values and can be directly determined by the thermal vibration of the atoms. Therefore, we say that the lowest possible temperature is 0 K, also known as absolute zero.
Despite the existence of Kelvin, other usual scales, based on other substances, such as Celsius
and Fahrenheit, continue to be used in the world. The figure below shows three thermometers graduated on the most common existing scales: Celsius,Kelvin and Fahrenheit:
thermometric scales
At scalesthermometric are used to measure temperature from some reference. Generally, two fixed points are taken to which the body or the reference substance would present the same properties such as volume, density, conductivity or electrical resistance, length, etc.
THE scaleCelsius it is the most used thermometer in the world. It is a centigrade scale, that is, it has 100 divisions of equal size between its fixed points, 0 ºC and 100 ºC, called degrees. As it is a usual scale, it admits negative temperatures: its absolute zero has a value of approximately -273.5 °C.
Lookalso: Thermometers and thermometric scales
THE scaleFahrenheit, in turn, it is used in a few countries, such as the United States and England. It was developed so that the point of Fusion of water is equal to 32°F. Thus, even reaching low temperatures, it is unlikely that negative temperatures are observed in countries that use this scale. the temperature of boiling of water in Fahrenheit is 212°F.
THE scaleKelvin was based on the thermal agitation of helium atoms in such a way that, when they reach total rest, these atoms are assigned a temperature of 0 K. Today, we know that this very low temperature is actually unreachable.
To convert temperature values expressed in one of the scales mentioned above, we can use the following equations:
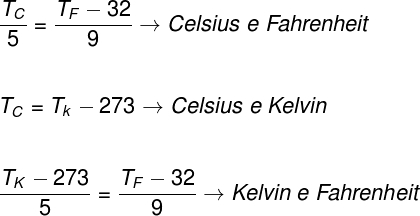
TK – temperature in Kelvin
TF – temperature in Fahrenheit
TÇ – temperature in Celsius
Heat
we say that heat is the thermal energy transferred between bodies that meet in temperaturesmany different, therefore being a form of energy. Furthermore, heat always passes from the higher temperature body to the lower temperature bodies, until thermal equilibrium is established.
Heat can be transmitted through three processes:
Driving: heat transmission through contact with surfaces;
Convection: heat transmission due to the formation of convective currents in a fluid;
Irradiation: heat transmission by electromagnetic waves.
Lookalso:Heat propagation processes
There are only two forms of heat: heatlatent and heatsensitive:
Heatsensitive: is the form of heat responsible for the change in temperature in a body. When a body receives sensible heat, its temperature rises; when the same body gives up sensible heat, its temperature drops.
Heatlatent: it is the amount of heat that must be transferred for a body or substance to change its physical state. When a body is at boiling or melting temperature, for example, its temperature does not change, even if it remains exposed to a heat source. There are no heat changes when a body exchanges latent heat, just changes in physical states. That's why we say he receives heatlatent.
Lookalso: Differences between sensible heat and latent heat
Thermal expansion
THE dilationthermal it occurs when a body receives or gives away large amounts of heat. Besides the changeintemperature or yours stateinaggregation (physical state), the transfer of heat to a body can cause changes in its dimensions. Thermal expansion depends on the temperature variation suffered by the body, in addition to its coefficient of expansion linear,shallow and volumetric.
According to the shape of the body, it is possible to determine which of its dimensions is more favored. For example, a needle has an elongated shape, so the most important dilation in this case is the linear. Altogether, there are three forms of thermal expansion:
Linear dilation: change in the length of a body. It depends on its coefficient of linear expansion (α).
Superficial dilation: change undergone by the area of a body. It depends on the coefficient of surface expansion (β).
Volumetric dilation: change occurred in the volume of a body. It depends on the volumetric expansion coefficient (γ).

Expansion joints are used so that the railroad bars do not expand and, consequently, do not bend.
Lookalso:Thermal expansion of solids
Thermodynamics
THE Thermodynamics is an important area of Thermology that studies the relationships between heat,work,temperature and other quantities, like pressure,volume, etc. It is responsible for establishing laws that govern all transformations that can be undergone by matter, such as the law of energy conservation, also known as the first law of thermodynamics.
Lookalso:Fundamentals of Calorimetry
Learn about the laws of thermodynamics and a brief description of its content:
Zero Law of Thermodynamics: is the law of thermal equilibrium. This law says that all bodies tend to exchange heat until they reach thermal equilibrium.
First Law of Thermodynamics: is the law of conservation of energy. This law states that all the heat received by a system during a thermodynamic process can be converted into work or into an increase in its internal energy.
Second Law of Thermodynamics: is the law of entropy. This law states that all systems that receive heat tend to reach lower and lower levels of organization.
Third Law of Thermodynamics: is the law of absolute zero. This law tells us that absolute zero is actually unattainable. No matter how cold a body is, it will never be at 0 K.
Thermology Formulas
Check out some Thermology formulas that may be useful for your study:
Conversion of thermometric scales
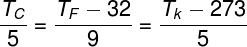
-
Sensitive heat calculation

Q – sensible heat
m - pasta
ç – specific heat
ΔT - temperature variation Calculation of latent heat
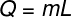
Q – heat
m - pasta
L – latent heat
-
linear thermal dilation
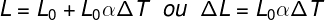
L – final length
L0 – initial length
ΔT - temperature variation
α – linear expansion coefficient -
surface thermal dilation
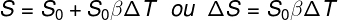
s – final area
s0 – initial area
ΔT - temperature variation
β – coefficient of surface expansion -
Volumetric Thermal Dilation
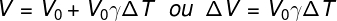
V - Final Volume
L0 – initial volume
ΔT - temperature variation
γ – volumetric expansion coefficient
First Law of Thermodynamics
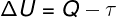
ΔU – internal energy variation
Q – heat
τ - work
Summary
Temperature: the hotter a body is, the greater the vibration of its molecules. Such agitation is called temperature.
Heat: when two bodies of different temperatures meet in thermal contact, heat is transferred from the higher temperature body towards the less hot body
Scalesthermometric: are used to represent temperatures in different units, such as Celsius and Fahrenheit.
Dilationthermal: when a body receives heat and experiences increases in temperature, its dimensions can increase. This effect is called thermal expansion.
See too: What is the difference between heat and temperature?
Thermology Exercises
1) A thermometer calibrated on the Fahrenheit scale indicates a temperature of 68°F. What is the value of this temperature on the Celsius scale?
Resolution
to convert Fahrenheit in Celsius, we will use the formula below:

2) A body with 10 g of specific heat equal to 1.2 cal/g °C is subjected to a temperature variation of 25 °C. Determine the amount of heat transferred to this body during the process.
Resolution
The exercise statement states that there was a variation in temperature for this body. Therefore, we will use the formula that calculates the amount of sensible heat:

Taking the data provided by the exercise, we will have to:

3) In a thermodynamic process, 500 cal is needed to melt a body with a mass equal to 10 g, which is in a solid state, at its melting temperature. Determine the latent heat of fusion of this body.
Resolution
To make the calculation you ask for, we will use the latent heat formula:
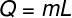
Using the data that was informed, we will have to:

4) Check the alternative that presents the name of the process of heat transmission by electromagnetic waves:
a) Driving
b) Convection
c) Transmission
d) Irradiation
e) Dilation
Resolution
The transmission of heat through electromagnetic waves is called irradiation. Through this process, the Sun is able to heat the Earth's surface.
5) A homogeneous metal bar with a length equal to 1.5 m is heated until its temperature of 25 °C reaches 150 °C. Considering that the coefficient of linear expansion of this bar is 1.2.10-5 °C-¹, determine the final length of the bar after heating.
Resolution
The type of dilation suffered by a bar is linear. Therefore, to calculate the final length of this bar, we will do the following calculation:
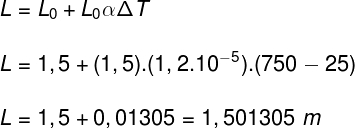
By Me. Rafael Helerbrock

