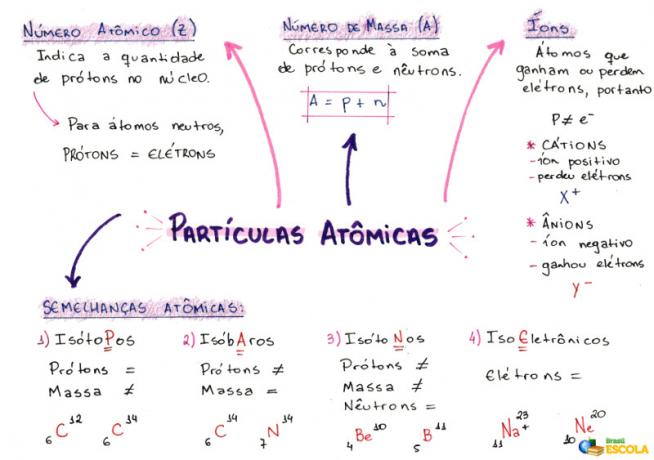
O calculating the number of atomic particles is used to indicate the amount of protons (in the nucleus), electrons (in the electrosphere) and neutrons (in the nucleus) present in any atom or ion. To do this, it is essential to know some characteristics of atoms:
1- Atomic number (Z)
It is a mathematical code, represented by the capital letter Z, positioned on the lower left side of an atom's abbreviation:
ZX
It indicates the number of protons (p) in the nucleus and the number of electrons (e) in the electrosphere of an atom. So, in general terms:
Z = p = e
2- Mass number (A)
It is a mathematical code that corresponds to the sum of the number of protons (p) and neutrons (n), both present in the nucleus of any atom. The equation representing the mass number is given by:
A = p + n
Since the number of protons is equal to the atomic number, we can write the equation to calculate the mass number as follows:
A = Z + n
If we know the mass number and atomic number of an atom, we can determine the number of neutrons as follows:
n = A - Z
3- ions
They are atoms that lose or gain electrons. They have a positive or negative sign positioned at the top right of their representation, as in the following model:
X+ or X-
Positive ion: This is called a cation and the positive sign indicates that it has lost electrons.
Negative ion: This is called an anion and the negative sign indicates that it has gained electrons.
Do not stop now... There's more after the advertising ;)
4- Atomic Similarities
a) Isotopes
Atoms that have the same atomic number and different mass numbers. Example:
7X14 and 7Y16
Atoms X and Y have the same atomic number (to the left of the acronym), that is, equal to 7. Atom X has a mass number (on the right of the acronym) equal to 14, and atom Y has a mass number equal to 16.
b) Isobars
Atoms that have the same mass number and different atomic numbers. Example:
15X31 and 13Y31
Atoms X and Y have a mass number (to the right of the acronym) equal to 31. Atom X, on the other hand, has an atomic number equal to 15, and atom Y has an atomic number equal to 13.
c) Isotones
Atoms that have different mass numbers and atomic numbers, but the same number of neutrons.
d) Isoelectronics
Atoms that have the same number of electrons. Example:
12X+2 and 7Y-3
Atom X has an atomic number equal to 12 and is a cation (with a positive charge +2), so it loses two electrons, thus having 10 electrons in its electrosphere. Atom Y, on the other hand, has an atomic number equal to 7 and is an anion (with a negative charge -3), so it gains three electrons, thus having 10 electrons in its electrosphere.
Mind Map: Atomic Particles
* To download the mind map in PDF, Click here!
Examples of calculating the number of atomic particles
Example 1: Determine the number of protons, neutrons and electrons in the atom 14X29.
The following values for the atom X were given:
Mass number (upper right) = 29
Atomic number (bottom left) = 14
To determine the number of protons:
The number of protons is always equal to the atomic number, so the X atom has 14 protons.
To determine the number of electrons:
As the atom X is not an ion, therefore, the number of electrons is equal to the number of protons, that is, 14.
To determine the number of neutrons:
The number of neutrons is determined using the number of mass and protons in the following formula:
A = p + n
29 = 14 + n
29 - 14 =n
n = 15
Example 2: Determine the number of protons, neutrons and electrons of ion X+3, knowing that their mass number and atomic number are, respectively, 51 and 23.
The following values for ion X were given:
Mass number = 51
Atomic number (bottom left) = 23
To determine the number of protons:
The number of protons is always equal to the atomic number, so the X atom has 23 protons.
To determine the number of electrons:
The ion X is positive (+3), so it is a cation that has lost three electrons. So its number of electrons is 20.
NOTE: The reduction or increase in the number of electrons always occurs in relation to the atomic number.
To determine the number of neutrons:
The number of neutrons is determined using the number of mass and protons in the following formula:
A = p + n
51 = 23 + n
51 - 23 =n
n = 28
Example 3: An atom W has an atomic number and mass equal to, respectively, 29 and 57, being isobare of a atom Y, which has an atomic number equal to 30, which is isotope of an atom B, whose mass number is 65. With this information, determine the number of protons, neutrons and electrons in atom B.
Data provided by the exercise:
Atom W
atomic number (bottom left) = 29
mass number (upper right) = 57
Y isobar, ie the mass of Y is also 57.
Y atom
atomic number = 30
mass number = 57
With these two values, we must determine its neutron number because it is isotone of element B:
A = Z + n
57 = 30 + n
57 - 30 = n
n = 27
Atom B:
mass number = 65
number of neutrons = 27
With these data, we must determine its atomic number, because with that, we will be determining its number of protons and its number of electrons (since it is not an ion):
A = Z + n
65 = Z +27
65 - 27 = Z
Z = 38
Therefore, the atom B has 38 protons, 38 electrons and 27 neutrons.
* Mind Map by Victor Ricardo Ferreira
Chemistry teacher
By Me. Diogo Lopes Dias