In the study of optical isomerism we come across several substances enantiomeric. Those enantiomers they are substances with the same molecular formula, but their atoms occupy spatial arrangements so that their structures are exactly mirror images of each other and are not superimposable.
An example of an enantiomeric substance widely used in the chemical industry is aspartame (C14H18N2O5).
This compound is 180 times sweeter than sucrose (sugar), that's why it is used in artificial sweeteners, with a smaller amount of it being added to the food to obtain the same sweet taste that is obtained with a greater amount of sugar. It is widely used as a sweetener in Brazil and worldwide because, in addition to being very sweet, providing the body with 4 g/cal, it does not have an unpleasant taste.
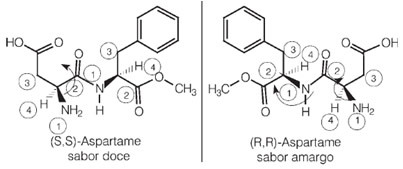
Aspartame was discovered in 1965 and its structure has two carbon atoms as asymmetric centers, and can therefore have four enantiomers. The figure below shows us that the (S, S)-aspartame form is the enantiomeric form that has a sweet taste, while the (R, R)-aspartame form, which is its optical isomer, has a bitter taste:
Do not stop now... There's more after the advertising ;)
However, the use of aspartame-based sweeteners presents a concern for people who have phenylketonuria, which is a metabolic alteration in which the person does not have the enzyme phenylalanine hydroxylase in the body. The use of these sweeteners is not recommended for these people because aspartame undergoes hydrolysis in the body, producing aspartic acid, methanol and phenylalanine. The latter is transformed by phenylalanine hydroxylase and, as they do not have this enzyme, phenylalanine will accumulate in the body, causing nervous system damage.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "The isomers of aspartame and their sweetening properties"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/os-isomeros-aspartame-suas-propriedades-adocantes.htm. Accessed on June 27, 2021.



