THE FirstLawgivesThermodynamics is an application of principlegivesconservationgivesenergy for thermodynamic systems. According to this law, the variation of internal energy of a thermodynamic system is the difference between the amount of heat absorbed by the system and the work done by it.
Lookalso:Fundamental concepts and summary of Thermology
What is the First Law of Thermodynamics?
The First Law of Thermodynamics is a direct consequence of the principle of energy conservation. According to this principle, the total energy of a systemalways remains constant, since she is not lost, but transformed.
Within the scope of Thermodynamics, are used more specific notions and less generic than those used in the principle of energy conservation. In the First Law of Thermodynamics, we use concepts like energyinternal,heat and work, which are relevant to the scope of Thermal machines (technological applications of fundamental importance for Thermodynamics).
Imagine a steam-powered machine, when the working fluid of that machine (water vapor) receives heat from an external source, two energy conversions are possible: steam can have its own temperature increased by a few degrees or, even, it can expand and move the pistons of that machine, thus performing a certain amount of work.
"The variation in the internal energy of a thermodynamic system corresponds to the difference between the amount of heat absorbed by it and the amount of work that this system performs."
Formula of the First Law of Thermodynamics
The formula used to mathematically describe the First Law of Thermodynamics is shown below:

U – internal energy variation (cal or J)
Q – heat (lime or J)
τ – work (lime or J)
In order to use this formula, we need to pay attention to some signal rules:
ΔU – will be positive if the system temperature increases;
ΔU – will be negative if the system temperature decreases;
Q – will be positive if the system absorbs heat from the external environment;
Q – it will be negative, if the system gives heat to the external environment;
τ – it will be positive if the system expands, carrying out work on the external environment;
τ – it will be negative if the system contracts, receiving work from the external environment.
internal energy variation
The term ΔU refers to the energy change attributed to the kinetic energy of the constituent particles of the system, in the case of an ideal gas, it can be said that ΔU is equivalent to:
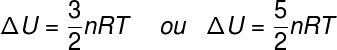
no – number of moles (mol)
R – universal constant of ideal gases (0.082 atm.l/mol. K or 8.31 J/mol. K)
T – absolute temperature (kelvin)
Analyzing the formulas, it can be seen that, if there is no temperature change in the system, its internal energy will also remain unchanged. Furthermore, it is important to say that for thermal machines, which operate in cycles, the variation of the internal energy, at the end of each cycle, must be null, because at that point, the engine returns to operating with the initial temperature.
Lookalso:Performance of thermal machines: how is it calculated?
Heat
Moving on to the next term, Q, which refers to the amount of heat transferred to the system, we usually use the fundamental equation of calorimetry, shown below:
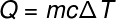
Q -heat (lime or J)
m – mass (g or kg)
ç – specific heat (cal/gºC or J/kg. K)
ΔT – temperature variation (celsius or kelvin)
Work
The last of the quantities related to the First Law of Thermodynamics is work (τ), which has a analytical formula only for transformations that occur under constant pressure, also known like isobaric transformations, watch:
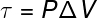
P – pressure (Pa or atm)
ΔV – volume variation (m³ or l)
When the pressure exerted on the system is not constant, the work can be calculated by the area of the graph of pressure versus volume (P x V). To learn more about this scalar magnitude, visit: work.
solved exercises
Question 1)(CefetMG) The work performed in a closed thermal cycle is equal to 100 J, and the heat involved in thermal exchanges is equal to 1000 J and 900 J, respectively, with hot and cold sources.
From the First Law of Thermodynamics, the variation of the internal energy in this thermal cycle, in joules, is
a) 0
b) 100
c) 800
d) 900
e) 1000
Resolution
Alternative a.
Let's solve the exercise using the First Law of Thermodynamics, note:

According to the statement, we are asked to calculate the variation of internal energy in a closed thermodynamic cycle, in which case we know that the internal energy variation must be zero, as the machine will return to operating at the same temperature as it was at the beginning of the cycle.
Question 2)(Upf) A sample of an ideal gas expands by doubling its volume during an isobaric and adiabatic transformation. Considering that the pressure experienced by the gas is 5.106 Pa and its initial volume 2.10-5 m³, we can say:
a) The heat absorbed by the gas during the process is 25 cal.
b) The work done by the gas during its expansion is 100 cal.
c) The internal energy variation of the gas is –100 J.
d) The gas temperature remains constant.
e) None of the above.
Resolution
Alternative c.
Using information provided by the exercise statement, we will use the First Law of Thermodynamics to find the correct alternative:
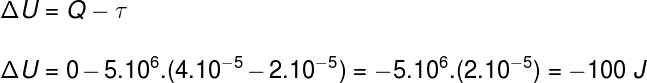
Question 3)(wow) A cooking tank contains high pressure gas. When we open this cylinder, we notice that the gas quickly escapes into the atmosphere. As this process is very fast, we can consider it an adiabatic process.
Considering that the First Law of Thermodynamics is given by ΔU = Q - W, where ΔU is the change in energy inside the gas, Q is the energy transferred in the form of heat and W is the work done by the gas, this is correct state that:
a) Gas pressure increased and temperature decreased.
b) The work done by the gas was positive and the gas temperature did not change.
c) The work done by the gas was positive and the gas temperature decreased.
d) The gas pressure increased and the work performed was negative.
Resolution
Alternative c.
Once the gas volume expands, we say that the work performed was positive, that is, the gas itself performed work on the external environment. Furthermore, since the process occurs very quickly, there is no time for the gas to exchange heat with the surroundings, so the following occurs:
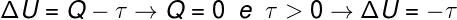
According to the calculation, the internal energy of the gas decreases by an amount equal to the work done by the gas, in addition, since there is a decrease in the internal energy of the gas, there is also a decrease in temperature.
Question 4)(Udesc) In a physics laboratory, experiments are carried out with a gas that, for thermodynamic analysis purposes, can be considered an ideal gas. From the analysis of one of the experiments, in which the gas was subjected to a thermodynamic process, it was concluded that all heat supplied to the gas was converted into work.
Check the alternative that represents correctly the thermodynamic process performed in the experiment.
a) isovolumetric process
b) isothermal process
c) isobaric process
d) adiabatic process
e) composite process: isobaric and isovolumetric
Resolution
Alternative b.
For all the heat supplied to a gas to be converted into work, there must be no absorption of internal energy by it, in other words, the gas needs to go through an isothermal process, that is, a process that takes place at temperature constant.
By Rafael Hellerbrock
Physics teacher
Source: Brazil School - https://brasilescola.uol.com.br/fisica/primeira-lei-da-termodinamica.htm
