When we studied the heat transmission by conduction we saw that this heat transfer process occurs through all the material through the exchange of energy between nearby particles, that is, between particles adjacent. The conduction mechanism occurs when molecules or atoms that are at a higher temperature transfer part of the energy to nearby molecules or atoms that are at lower energy. Thus, we say that energy is transferred from the high temperature region to the low temperature region. Thermal conduction aims at the thermal balance of the material.
We are now going to present the law that governs thermal conduction, also known as Fourier's law. It is named after the scientist who first studied in detail the transmission of heat by conduction.
In the figure above we have a metal bar connected to two containers, one containing boiling water and the other containing a mixture of water and ice. From the figure we see that the bar is laterally isolated.
Joseph Fourier, through experiments, managed to observe that the temperature varies linearly throughout the bar, that is, from one end to the other. Therefore, the heat flow
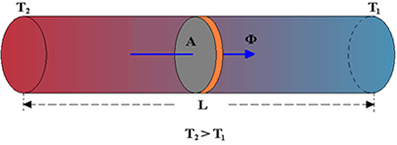
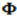 across the bar is proportional to the area of section A of the bar and the temperature difference, ΔT = Tf - Ti, between the two ends; and inversely proportional to the length, L, of the bar. See the figure below:
across the bar is proportional to the area of section A of the bar and the temperature difference, ΔT = Tf - Ti, between the two ends; and inversely proportional to the length, L, of the bar. See the figure below:
We can mathematically define that the heat flux is nothing more than the heat quotient Q transmitted from one face to another over a temperature range. So the heat flux is defined by:

Analytically, Fourier's law, or thermal conduction law, can be expressed by:

In the equation above, k is a material-dependent constant and is called thermal conductivity of material. The value of this coefficient is high for good heat conductors; and low for bad conductors, known as thermal insulators.
By Domitiano Marques
Graduated in Physics

