As the name says, transuranic elements are those that have an atomic number greater than the atomic number of uranium, that is, greater than 92 and, therefore, come after this element in the Periodic Table.
Obtaining and discovering these elements in the laboratory are due to experiments carried out with the bombardment with particles from stable atomic nuclei, from elements that are not naturally radioactive Thus, they undergo transmutation and transform into other elements.
The first attempts to produce elements other than uranium were made by Fermi, Segrè and collaborators in 1934, drawing on the work of Irene Curie and Frederic Joliot on artificial radioactivity through the bombing of cores.
However, it was not until 1940 that this was done for the first time, by Edwin M. McMillan and Philip H. Abelson. They bombarded the uranium-238 core with a neutron beam; and the result was the obtaining the first transuranic element, netunium (Np), with atomic number 93:
92238U + 01n → 93239Np + -10β
In this case, the neutrons have no charge, so their bombardment occurs more easily, not being repulsed by the nucleus, which is positively charged. However, as research to obtain transuranic elements deepened, other particles (such as alpha particles, deuterons and protons) came to be used as projectiles in these bombings. But since they have a positive charge, it is necessary to use a particle accelerator, which increases their speed in order to break the repulsion forces with the nucleus.
Thus, with the help of particle accelerators, it was possible to produce several artificial elements with higher atomic numbers. In the same year of 1940, another transuranic element was produced, the plutonium (Pu), with atomic number 94, according to the following reactions:
Do not stop now... There's more after the advertising ;)
12H+ 92238U → 93239Np + 2 01no
93239Np → 94238pu + -10β
The other transuranic elements discovered were: americium (Am), curium (Cm), berkelium (Bk), californium (Cf), einsteinium (Es) and fermium (Fm). And over time there were others. The table below shows their atomic numbers and the reactions of their obtainments:

However, the determination of the properties of these elements is very difficult, as they are obtained in small quantities and also present great nuclear instability, decaying rapidly the higher its atomic number.
A scientist who excelled in this field was Glenn T. Seaborg, who headed the section that worked with transuranic elements within the Manhattan Project (responsible for the development of the atomic bomb). It was he who isolated and discovered plutonium, along with E. M. McMillan, J. W. Kennedy and A. Ç. Wahl. Later, he also discovered four more transuranic elements and was also involved in discovering five more.
Glenn Seaborg, in 1944, proposed the hypothesis that elements with an atomic number above actinium (Z = 89) formed a new series similar to lanthanides. This allowed the explanation of the chemical properties of both already identified and unidentified elements. So, in 1945, he published the first Periodic Table that contained the newly discovered elements.
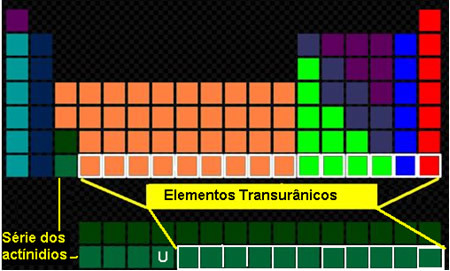
Location of transuranic elements in the Periodic Table
For his work in this area he received the Nobel Prize in Chemistry in 1951, together with physicist Edwin M. McMillan, cited above. In his honor, in 1997, the artificial element of atomic number 106 was named seaborgium.
By Jennifer Fogaça
Graduated in Chemistry