We are surrounded by equipment that, in order to function, needs cells or batteries. However, many of these portable devices are getting smaller and with that comes the great need for miniature batteries.
An example of this type of battery is the mercury one or also called mercury-zinc pile.
Every cell consists of two electrodes, the anode (negative pole) and the cathode (positive pole), and an electrolyte. In the case of the mercury cell, the anode is formed by a capsule of metallic zinc (Zn(s)) it's the cathode per mercury oxide II (HgO(s)). Both Zn and HgO are ground into powder and compacted to make the pile as small as possible. O electrolyte is made from a solution of saturated potassium hydroxide (KOH(here)).
Zn oxidizes, donating its electrons to HgO, as shown in the semi-reactions and global reaction of this cell below:
Anode Half Reaction: Zn(s) + 2 OH1-(here) → ZnO(s) + 2 H2O(1) + 2e-
Cathode Semi-Reaction: HgO(s) + H2O(1) + 2e- → Hg(1) + 2 OH1-(Theq)
Global Reaction: HgO(s) + Zn(s) → ZnO(s) + Hg(1)
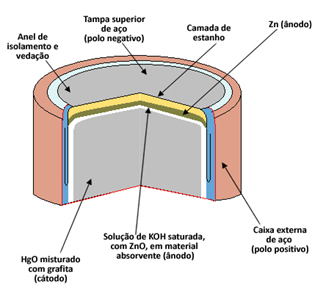
Mercury cells are used in digital watches, wristwatches, cameras, calculators, electronic organizers, hearing aids and other portable electrical devices that require efficient work and durability, as these batteries have voltage of 1.35V.

Unfortunately, improperly disposing of these batteries can pose serious risks to the environment as they contain mercury, which is a heavy metal. Mercury can contaminate soil, groundwater, lake and river water, and reach animals and man. It is toxic even in small amounts. Among the health problems that mercury can cause are: damage to the mucous membranes, skin, kidneys, violent nausea, vomiting, abdominal pain, bloody diarrhea, and it can lead to death.
To find out more about why batteries are toxic waste, how mercury is contaminated and what we should do with used batteries, read the texts cited below.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/pilhas-mercurio.htm


