THE stereoisomerism, also known as Space Isomerism, is a type of isomerism in which its isomers are differentiated by the bonds between their atoms being arranged differently in space.
There are two types of stereoisomers, the diastereoisomers and the enantiomers. Diastereoisomers are geometric isomers of the cis-trans type, which are not mirror images of each other, while enantiomers are optical isomers that are mirror images of each other.
Talking a little about the cis-trans diastereoisomer, it only occurs in compounds that are unsaturated or that are cyclic. Why doesn't it occur in saturated chain compounds, that is, which have only single bonds between carbons?
It can happen that we get confused, thinking that certain saturated compounds are diastereoisomers, when in fact they represent the same compound. For example, below, we have three spatial conformations of the atoms of 1,2-dichloroethane:
H H H H H Cl
│ │ │ │ │ │
H — Ç — Ç — H H — Ç — Ç — ClH — Ç — Ç — H
│ │ │ │ │ │
Cl ClCl H Cl H
Do not stop now... There's more after the advertising ;)
Are these three diastereoisomers? Do not. Actually, the three molecules are of the same compound. What happens is that the single bonds or sigma (σ) between carbons can undergo rotations, resulting in different atomic arrangements.
See how this happens in the scheme below with ethane:

These compounds, which are actually the same compound, do not carry out geometric cis-trans isomerism, but are called conformational isomers, as they differ only in the conformation of their atoms caused by rotation around the single bond.
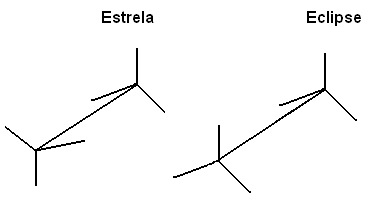
One of the ways to represent these molecules to make it easy to visualize the rotation of the connection axis is through the Newman's projection, in which it is considered as if the plane of vision were exactly on the axis of the bond between the carbons and they are represented by a central circle. See Newman's projection for ethane:

We can also represent these different conformations through the formula on easels:
This free rotation of the linkage axis no longer occurs in unsaturated compounds or in cyclics. For example, imagine that we build a simple model, representing two carbons by two Styrofoam balls connected by a toothpick stuck in them.
When we have a single toothpick and we hold one of the balls, the other can easily rotate around its own axis, just as it does with simple binding.

However, if we put one more toothpick connecting the two spheres, we won't be able to hold one sphere and rotate the other. If we do this, the toothpicks will break. Likewise, when there is a pi bond and a sigma (double bond) there is an impediment to the rotation of the carbons.
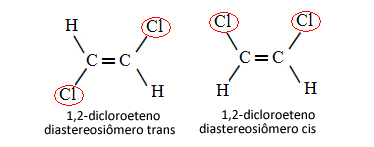
Therefore, in the case below, in which we have a double bond between the carbons, it is not a question of two conformational isomers, that is, two conformations for the same molecule; we actually have two cis-trans diastereoisomers:
See more about this type of isomerism in the text Geometric or cis-trans isomers.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Conformational Isomerism"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/isomeria-conformacional.htm. Accessed on June 28, 2021.