In the study of ideal gases we see that a gas is composed of atoms and molecules, which move according to the laws established by kinematics. In a gas, its particles are usually very far apart, having a void between them. We also see that the main characteristic of gases is that there is practically only interaction between their particles when they collide with each other.
Regarding the Ideal Gas Law, we can say that it shows us the relationship between pressure, volume, temperature and number of moles. This relationship is obtained from a simple model for gases, which makes it possible to determine the relationship between macroscopic quantities based on the study of the movement of atoms and molecules. The kinetic theory of gases is based on four postulates:
1 – the gas is formed by molecules that are in disorderly and permanent movement. Each molecule can have a different speed than the others.
2 – each gas molecule interacts with the others only through collisions (normal contact forces). The only energy of molecules is kinetic energy.
3 – all collisions between the molecules and the walls of the container containing the gas are perfectly elastic. The total kinetic energy is conserved, but the speed of each molecule can change.
4 – molecules are infinitely small. Most of the volume occupied by a gas is empty space.
Based on these postulates, Boltzmann and Maxwell show that the average kinetic energy of the total molecules of an ideal gas is proportional to the temperature, according to the expression:
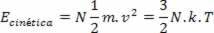
Where k is the Boltzmann constant and N is the number of molecules. The value of k can be calculated from the gas constant R and the Avogadro number NTHE per
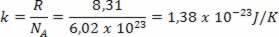
The expression obtained shows that the temperature is proportional to the average kinetic energy of the molecules of an ideal gas. Thus, we see that temperature is an average of the degree of agitation of the molecules in a gas. Using the number of moles, we have:
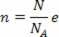
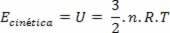
By Domitiano Marques
Graduated in Physics
Source: Brazil School - https://brasilescola.uol.com.br/fisica/teoria-cinetica-dos-gases.htm

