Organometallic are organic compounds that have at least one metal atom bonded to a carbon atom. Usually the metals that form this type of substance are: magnesium (Mg), zinc (Zn), lead (Pb) and mercury (Hg).
Among the most common organometallics are the organomagnesium, better known as Grignard compounds or reagents, whose metal bonded to carbon is magnesium, and it, in turn, is bonded to a halogen, which can be one of the following elements of family 17 of the Periodic Table: fluorine (F), chlorine (Cl), bromine (Br) and iodine (I).
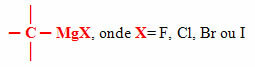
Organometallic compounds are normally toxic, as they have metals that accumulate in the body and are more soluble in organic compounds than other inorganic compounds that have the presence of these metals. An example where this can be seen is when miners or industries throw organometallic compounds into lakes and rivers. The results are serious problems for the health of users of these waters (both humans and animals), as well as other ecological problems.
Below are three examples of organometallic compounds present in our daily lives:
• Ethyl mercury chloride (H3C CH2 ─ HgCl): this compound can be used as a fungicide in seed preservation. However, its use is very dangerous, as, as it contains mercury, it is highly toxic, especially for the nervous system;
• Butyl-lithium (H3C CH2 CH2 CH2 ─ Li): this compound is used as a polymerization initiator for the production of elastomers, that is, polymers with elastic properties;
• Tetraethyl-lead (or tetraethyl lead): for a long time this compound was used as an antiknock in gasoline, as it increased its octane rating and engine efficiency. However, along with the burning of gasoline, this compound released lead into the environment, causing atmospheric pollution. This lead is toxic, pollutes the environment and is especially dangerous because it affects the brain, causing effects on motor coordination.
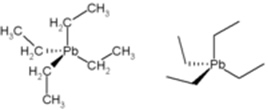
Structure of Tetraethyl-lead.
Thus, in its place another organometallic is being used: ferrocene, which has in its structure two cyclopentadienyl rings on each side of an iron as shown in the chemical structures bellow:
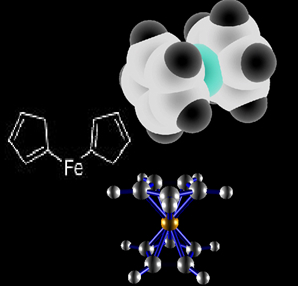
Ferrocene structure.
The nomenclature of organometallics follows the following scheme:

Examples:
CH3
│
CH2
│
H3C ─ CH2 ─Al: triethylaluminum
│
CH2
│
CH3
H3C ─ CH2 ─ CH2 ─ Li: propyllithium
If it is a Grignard compound, we have:

Examples:
H3C CH2 ─ MgCl: Ethyl Magnesium Chloride
H2C ═ CH ─ MgBr: Vinyl Magnesium Bromide
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/compostos-organometalicos.htm
