Electrolysis is a process that has wide industrial application and, therefore, its quantitative aspects are extremely important for factories. For example, they need to know how much reagent to use, how long to carry out the process, and how much of the desired product they are going to get.
Through the igneous electrolysis of sodium chloride (table salt), industries produce chlorine gas, so they need to know what volume of chlorine gas they will be able to obtain.
In addition, several metal parts undergo electrolysis in an aqueous medium in order to be coated with another metal, as in the case of gold or silver semi-jewels and costume jewelry. The color quality of the coated object and the effectiveness of protection against its corrosion depend, among other things, on the time of electrolysis and the intensity of electrical current used.
Thus, the English physicist and chemist Michael Faraday (1791-1867) began to study these aspects involving electrolysis and after several experiments he discovered some laws in that case.

Michael Faraday (1791-1867)
One of them showed that the amount of mass of a metal that is deposited on the electrode is directly proportional to the amount of electrical charge (Q) that passes through the circuit.
The electric charge (Q) is given by the following formula:

On what:
i = electric current intensity (unit: ampere - A)
t = time (unit: seconds – s)
So the unit of charge would be A. s, which is equal to the coulomb unit (C).
Do not stop now... There's more after the advertising ;)

In the year 1909, physicist Robert Andrews Millikan (1868-1953) determined that the electric charge of 1 electron is equal to 1.602189. 10-19 Ç.

Robert Andrews Millikan (1868-1953)
Avogadro's constant says that in 1 mole of electrons there are 6.02214. 1023 electrons. Thus, the amount of charge carried by the passage of 1 mol of electrons is equal to the product of the electric charge of each electron by the amount of electrons we have in 1 mol, that is:
1,602189. 10-19 Ç. 6,02214. 1023 = 96486 C
Therefore, if we know the amount of matter (n) that travels through the circuit, just multiply by the value that we just saw that we found the electrical charge (Q) that will be needed to carry out the electrolysis process that if you want:

This value (96486 C) is known as Faraday's constant (1F). Thus, if the charge used in the process is given in faraday, then we can use relations established by rules of three and calculate the amount of mass that will be deposited in the electrolysis.

Read the text Applications of Quantitative Aspects of Electrolysis to know exactly how these calculations can contribute to solving problems related to electrolysis processes and even batteries.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Quantitative Aspects of Electrolysis"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/aspectos-quantitativos-eletrolise.htm. Accessed on June 28, 2021.
Chemistry

Applications of Electrolysis, electroplating, nickel plating, chrome plating, nickel, chromium, cathode, sodium, aluminum, chlorine, caustic soda, hydrogen gas, igneous electrolysis, aqueous electrolysis, alkali metals, alkaline earth, gas chlorine.
Chemistry
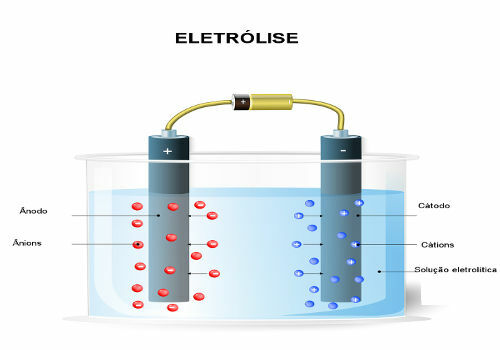
Electrolysis, electrolyte solutions, electric current, oxidation-reduction reactions, spontaneous chemical process, chemical process non-spontaneous, transformer, artificial transformation, industries, alkali metals, alkaline earth, hydrogen gas, gas cl

