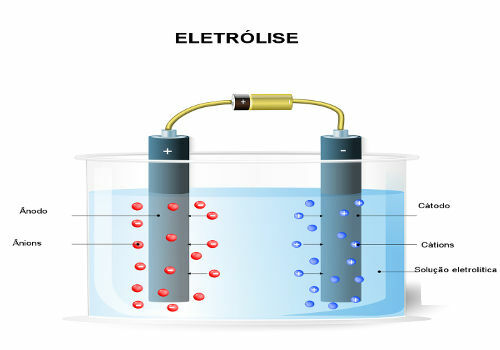
Electrolysis is generally studied in Electrochemistry as a system that contains a vat or electrolytic cell (a container) with a liquid substance or in solution, in which two electrodes are immersed (the cathode or negative pole and the anode or pole positive). Such electrodes are connected to a generator (cell or battery) which, when turned on, conducts electricity from a electrode to another through the liquid, causing oxidation-reduction reactions that transform electrical energy into energy chemistry.
However, when electrolysis is applied in industries, in practice it is not just an electrochemical cell with two electrodes; but rather several huge tanks connected in series, as shown in the opening image. In addition, only one generator with enough capacity is used to service all these tanks, because if a generator were used for each tank, the economic loss would make production unfeasible industrial.
In the text Quantitative Aspects of Electrolysis it was shown that by means of the electric charge formula (Q = i. t) and through the relation of the Faraday constant (96500 C) with the molar masses of the substances and with the half-reactions balanced cathodic and anodic, it is possible to determine the mass of the substance that has been transformed or obtained in a vat electrolytic.
This can also be done in the case of series electrolysis. But there are two factors that must be taken into account:
1. Since the generator is one for all the electrolytic cells, the time (t) and the intensity of the electric current (i) will be the same for all the cells. Therefore, the electrical charge (Q) will also be the same for all cells;
2. The mass obtained or transformed in each cell will be different, since the substances contained in each one are distinct. This is because, for example, the Zn ion2+ requires twice as many electrons as the Ag ion1+. These masses can be calculated using rules of three or directly using the formula below:
m = __M. Q__
q. 96500
On what:
M = molar mass of each substance;
Q = electrical charge of the system;
q = ion charges, eg if the ions are Ag1+, the value of q will be 1.
See an example of how to perform this type of calculation:
Example: There are three electrolytic vats connected in series, each containing AgNO3, CuSO4 and ZnCℓ2. Knowing that 108 g of metallic silver were deposited in the first vat, it can be concluded that the following were also deposited:
a) 31.75 g of metallic copper.
b) 65.4 g of metallic zinc.
Do not stop now... There's more after the advertising ;)
c) 63.5 g of metallic copper.
d) 108 g of metallic copper.
e) 108 g of metallic zinc.
(Atomic masses: Ag = 108; Cu = 63.5; Zn = 65.4).
Resolution:
From the mass found in the first electrolytic cell, we can discover the electrical charge of the system, which is the same for all cells:
Ag+ + 1e-→ Ag
↓ ↓
1 mol 1 mol
1mol. 96500 C 108 g (molar mass)
Q 108 g (obtained mass)
Q = 96500C
With this value in hand, we can discover the masses of other metals. This can be done through the rule of three or the formula that was given earlier:
- By rule of three:
2nd electrolytic bowl: 3rd electrolytic bowl:
Ass2+ + 2e-→ Cu Zn+2 + 2e-→ Zn
↓ ↓ ↓ ↓
2 mol 1 mol 2 mol 1 mol
2. 96500 C 63.5 g 2. 96500 C 65.4 g
96500 cmAss 96500 cmZn
mAss = 31.75 gmZn = 32.7 g
- By the formula: m = __M. Q__
q. 96500
2nd electrolytic bowl: 3rd electrolytic bowl:
mAss = (63,5). (96500) mZn = (32,7). (96500)
2. 96500 1. 96500
mAss = 31.75 gmZn =32.7 g
Therefore, the correct alternative is the letter “a”.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Serial Electrolysis"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/eletrolise-serie.htm. Accessed on June 28, 2021.
Chemistry

Applications of Electrolysis, electroplating, nickel plating, chrome plating, nickel, chromium, cathode, sodium, aluminum, chlorine, caustic soda, hydrogen gas, igneous electrolysis, aqueous electrolysis, alkali metals, alkaline earth, gas chlorine.
Chemistry
Electrolysis, electrolyte solutions, electric current, oxidation-reduction reactions, spontaneous chemical process, chemical process non-spontaneous, transformer, artificial transformation, industries, alkali metals, alkaline earth, hydrogen gas, gas cl