Thermal machines are devices that convert partially thermal and chemical energy contained in its substances, such as heated gases, water vapor or gasoline, in work. Example: all types of internal combustion engines and steam powered machines.
Performance of thermal machines
O performance of thermal machines is a dimensionless quantity given by the ratio between the amount of mechanical work extracted from a thermal machine and the amount of energy supplied to it in the form of heat.
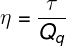
The greater the performance of a machine, the greater its efficiency. In other words, an efficient thermal machine is one that produces the most work consuming less amount of energy resources. You internal combustion engines, used to drive vehicles like motorcycles, cars, trucks and even aircraft, are designed to be increasingly efficient and pollute less. These thermal machines use as an energy source the heat obtained by burning fossil fuels as Gasoline, diesel oil, alcohol or aviation kerosene. Learn more about the history of thermal machines by clicking on here.
See too: Fossil fuels
Furthermore, the thermodynamic transformations undergone by the working substance are cyclic, that is, at the end of each cycle, they return to the same values of Pressure, Volume and Temperature. As a result, and in accordance with the 1st Law of Thermodynamics, the work obtained by a thermal machine is given by the difference between the amount of heat supplied for the operation of the machine (Qwhat) and the amount of heat the machine rejects (Qf) for its cold source (which could be the external medium):
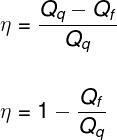
See too: Exercises on thermal machines
Mind Map: Thermal Machines

*To download the mind map in PDF, Click here!
The engines that use the diesel cycle have the highest yields of thermal machines - about 30% – while gasoline engines, which generally use the Otto cycle, have income of up to 20%.

Yield of ideal thermal machines
The ideal thermal machines, also known as thermal machines. carnot, are theoretical and operate according to the Carnot cycle. Carnot's cycle has four steps: two isothermal transformations it's two adiabatic transformations. These transformations are, respectively, extremelyslow and extremelyfast, making this cycle impractical in machinesreal. However, real machines are designed so that their operating cycle closely resembles the cycle of an ideal machine, in order to have the highest possible output.
The efficiency of ideal thermal machines can be calculated by the following equation:
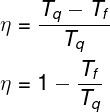
In the equation presented above, Tf is the temperature of the cold source, also called the chiller or sink, and Twhat is the temperature of the hot source. Importantly, the temperatures involved in these calculations must always be expressed in Kelvin.
See too: Thermometric scales and their conversions
By Rafael Hellerbrock
Graduated in Physics
Source: Brazil School - https://brasilescola.uol.com.br/fisica/rendimento-das-maquinas-termicas.htm
