Tonoscopy is joint ownership that studies the lowering of the maximum steam pressure of a given solvent due to the dissolution of a non-volatile (molecular or ionic) solute. The other colligative properties are:
Ebulioscopy
cryoscopy
osmoscopy
From the definition set out above, it is evident that, to really understand what is tonoscopy, It is essential to know three other concepts:
It is the force exerted by the steam of a certain liquid on the walls of a closed container when the evaporation rate is equal to the condensation rate.
In a container with a certain amount of ethanol, which evaporates under the influence of the temperature of the environment, as the steam meets the walls of the container, it ends up passing to the state liquid. Over time, the rate of evaporation becomes equal to the rate of condensation. At this point, the force that the steam exerts on the vessel walls is called the maximum steam pressure.
Mental Map: Tonometry or Tonoscopy

* To download the mind map in PDF, Click here!
Dissolution
It is the ability of a solvent to dissolve a certain solute. After dissolution, solute and solvent start to establish an intermolecular interaction with each other, that is, they are interconnected.
non-volatile solute
It is a material that has a high boiling point, that is, it cannot turn into a gas at ambient temperatures, for example. Thus, when added to a solvent, there will be no loss of this material to the environment in the form of gas.
O non-volatile molecular solute is the one that not able to undergo ionization or dissociation when dissolved in a solvent. So, if we add a molecule of this solute to the solvent, it will be just in the middle.
Youionic non-volatile olute é who suffers from the phenomenon of dissociation or ionization, that is, the breaking of bonds between the atoms occurs in it, which causes the fractionation of the molecular unit. If we dissolve 1 mole of sulfuric acid (H2ONLY4) in water, for example, we will have the presence of two moles of the hydronium cation and one mole of the sulfate anion in the middle, as shown in the equation below:
H2ONLY4 +2H2O → 2H+ + OS4-2
Do not stop now... There's more after the advertising ;)
By clarifying these basic concepts, it becomes easier and clearer to understand Tonoscopy.
Understanding Tonoscopy
To understand tonoscopy, let's briefly study the behavior of liquids such as water and ethanol. The boiling points of these two solvents are respectively 100OC and 78OÇ. Therefore, ethanol evaporates faster than water when they are at the same temperature and in the same amount.
If there is 50 mL of ethanol in one container and 50 mL of water in another container, for example, both closed and 250OC, the maximum steam pressure in the ethanol container will be higher because the amount of steam is higher inside.
graphically speaking, whenever the curve of a liquid is farther from the y (vertical) axis, the lower its maximum vapor pressure will be, as in the graph below:
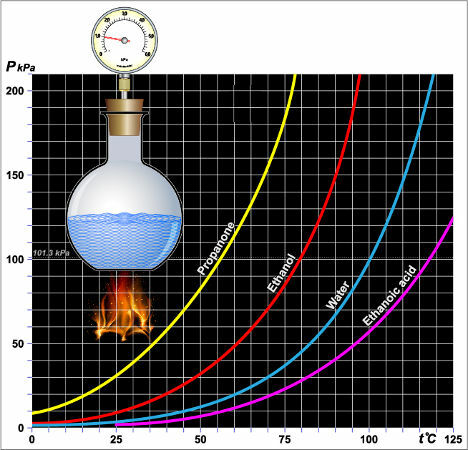
Vapor pressure of different liquids
Graphic subtitle: Propanone = propanone (acetone)
Ethanol = ethanol
water = water
Ethanic acid = Ethanoic acid
In the graph, we can confirm that the vapor pressure of ethanol (red curve) is always greater than that of water (blue curve) at any temperature.
NOTE: In short, the higher the boiling point of a given solvent, the lower its maximum vapor pressure and vice versa.
Like tonoscopy studies the decrease in maximum vapor pressure due to the dissolution of a non-volatile solute in the solvent, if we mix sodium chloride (NaCl) in water, the maximum water vapor pressure, at 100OC, which is 760 mmHg, will definitely decrease. But why does this happen?
When sodium chloride (ionic solute) dissolves in water, its ions interact with water molecules. This interaction makes solvent evaporation more difficult. As evaporation has been hampered, there will be less water vapor in the container, which will cause the maximum vapor pressure to decrease.
Thus, the greater the amount of sodium chloride in the same amount of water, the more difficult it will be to evaporate and the lower the maximum vapor pressure.
By Me. Diogo Lopes Dias
Chemistry

Dissociation and Ionization, Italian Scientist Volta, Electric Current, Swedish Physical Chemist Svant August Arrhenius, Theory of Arrhenius, positive ions, cations, negative ions, anions, caustic soda, table salt, polar molecules, dissociation ionic,
Chemistry

Colligative properties, tonoscopy, ebullioscopy, cryoscopy, osmoscopy, colligative effects, reduction of chemical potential of solvent, boiling temperature, melting point drop, osmotic pressure, non-volatile solute, solute, solvent, tempe