The electrolysis of water consists of the decomposition of this substance by means of an electric current and the addition of an electrolyte. Let's better understand how this happens?
Read too: What is electrolysis?
How does water electrolysis happen?
Water molecules are capable of self-ionization, generating H ions+ (or H3O+) and oh-:
H2O ↔ H+ + OH-
or
2 hours2O ↔ H3O+ + OH-
However, water is a very weak electrolyte and despite having these ions, it cannot conduct electric current. Thus, to carry out its electrolysis, that is, its decomposition by means of an electric current, it is necessary to add an electrolyte, an ionic solute that can be a salt, a base or an acid.

Priority order for selective discharge
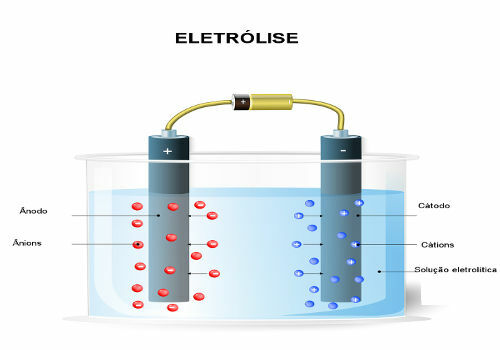
However, as explained in the text Aqueous electrolysis, in this case, we will not only have the ions coming from the water, but also those of the substance that was dissolved in it. In electrolysis, only one cation and one anion are discharged at the electrode, that is, it is a
selective discharge following a priority order.Thus, in order for the cathode and anode that will be discharged to be those of the water, and not those of the dissolved substance, it is necessary to choose a acid, a base or a salt whose ions are less easily discharged from the electrodes than the ions in water. To do this, we need to consult the priority queue shown below:
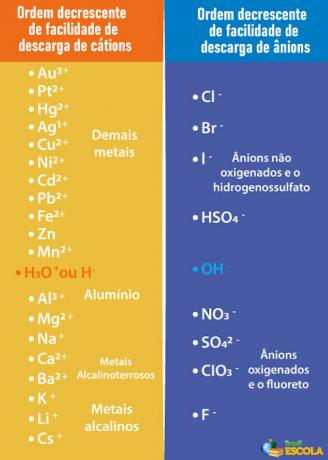
Note that the cations listed below the H+ have less ease of unloading than it. In the table on the right, we see that the anions below the OH- have less ease of unloading. Therefore, we can choose, for example, a salt, a base or an acid that forms the Na ions.+,K+, AT THE3-, ONLY42- and so on, in addition to also forming the same ions as water, that is, H+ and oh-. Some examples are: sulfuric acid (H2ONLY4), sodium hydroxide (NaOH) and potassium nitrate (KNO3).
Do not stop now... There's more after the advertising ;)
Example with the reactions that occurred in the electrolysis of water
Let's say that an electrolysis of water is carried out with the addition of sulfuric acid. In this case, we will have the formation of the following ions in the middle:
Acid dissociation: 1 H2ONLY4 → 2 H+ + 1 SO42-
Autoionization of water: H2O → H+ + OH- or 2 hours2O → H3O+ + OH-
Note that the only existing cation is H.+, so it is he who will suffer reduction (gain of electrons) on the negative electrode (cathode) and will produce hydrogen gasO (H2).
Now, speaking of anions, there are two anions in the middle, which are the OS42- and the oh-. As the table above shows, the OS42- it is more reactive and less easy to discharge. Thus, the OH- will be discharged, oxidizing (losing electrons) in the positive electrode (anode) and will produce gas oxygen(O2):
Cathode half-reaction: 4 H3O+ + 4 and- → H2O+H2
Anode half-reaction: 4 OH- → 2 H2O + 1 O2 + 4 and-
Adding up this entire process, we arrive at the global equation:
Water ionization: 8 H2O → 4 H3O+ + 4 OH-
Cathode half-reaction: 4 H3O+ + 4 and- → 4 H2O + 2 H2
Anode half-reaction: 4 OH- → 2 H2O + 1 O2 + 4 and-
Global equation: 2H2O → 2 H2 + 1 O2
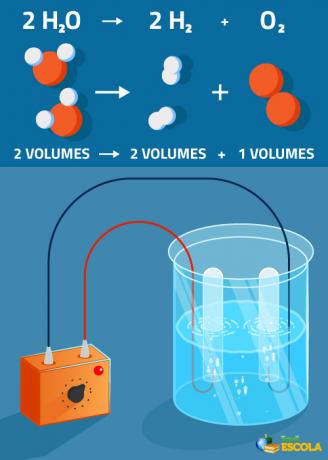
Note that the volume of hydrogen produced is twice that of oxygen. However, in practice, this strict ratio is not verified because oxygen is more soluble than gas hydrogen.
Read too: Obtaining aluminum through electrolysis
Applications of water electrolysis
The electrolysis of water is a very important process, considering that hydrogen is a gas that can be used as fuel. Like the petroleum-derived fuels are not renewable, hydrogen gas could become an important alternative.
In addition, there are already gasoline production methods that use the water electrolysis process. See how this is done in the text Scientists are able to transform carbon dioxide into gasoline.
By Jennifer Fogaça
Chemistry teacher
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Electrolysis of water"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/eletrolise-agua.htm. Accessed on June 28, 2021.
Chemistry

Applications of Electrolysis, electroplating, nickel plating, chrome plating, nickel, chromium, cathode, sodium, aluminum, chlorine, caustic soda, hydrogen gas, igneous electrolysis, aqueous electrolysis, alkali metals, alkaline earth, gas chlorine.
Chemistry
Electrolysis, electrolyte solutions, electric current, oxidation-reduction reactions, spontaneous chemical process, chemical process non-spontaneous, transformer, artificial transformation, industries, alkali metals, alkaline earth, hydrogen gas, gas cl

