Positrons are positively charged electrons.
Anything different in this definition? Of course yes, electrons usually have a negative charge. But then, how do you explain the existence of such particles?
Positron Formation
An electron is positively charged when a proton present in the nucleus decays into a neutron. Therefore, a positron is emitted from the nucleus when a positive particle (proton) collides with a neutral one (neutron).
Potassium isotope K-40 is considered a positron emitter.
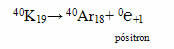
Reaction products: isotope of the element Argon (Ar-40) and positron 0℮+1 (electron with a positive charge).
The best term to define a positron would be “antimatter? If a positron collides with an electron, the two particles will be destroyed and a release of energy will occur, that is, it will be as if they never existed.
Do not stop now... There's more after the advertising ;)
Positron Application
Medicine is relying on devices that use these particles for new scientific discoveries.

Positron emission tomography (PET) and functional magnetic resonance imaging (FMRI) make it possible to observe, in detail and in real time, the functioning of the human brain. This technology allows us to perceive, for example, the influence of emotions on the process of falling ill.
By Líria Alves
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
SOUZA, Líria Alves de. "Positrons"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/positrons.htm. Accessed on June 27, 2021.

