Kc is the acronym that represents the equilibrium constant in terms of concentration of a particular chemical reaction. It is used whenever we refer to the establishment or not of equilibrium in a chemical reaction. For this, it is always necessary to perform a Kc calculation, which uses the concentrations in quantity of matter of the reaction participants.
THE Kc interpretation is very simple, because when:
Kc = 1, the reaction system is in equilibrium;
Kc > 1, the system is not in equilibrium and the direct reaction is predominating;
Kc < 1, the system is not in equilibrium and the reverse reaction is predominating.
Importantly, the interpretation of Kc depends on knowledge about chemical balance.
We have one chemical balance when a chemical reaction is reversible, that is, it presents a direct reaction (reactants form the products) and inverse reaction (products form the reactants), and the forward reaction speed is exactly the same as the reverse reaction speed. See an example:

In the example above, we have a chemical equilibrium (indicated by the two arrows), as there is a
direct reaction (A +B forming C+D) and the reverse reaction (C+D forming A+B). The coefficients a, b, c, d make the equation balanced.Each of the participants in a reaction always has a concentration in quantity of matter (mol/L). With the concentration values, we can calculate the Kc of the reaction. However, to perform this calculation, it is necessary to assemble the Kc expression.
The assembly of the Kc expression always uses the same pattern. With this pattern, we multiply the concentrations of products raised to their respective exponents and divided by the multiplication of reactants raised to their respective exponents.
The Kc expression for the generic balance below would be:

Do not stop now... There's more after the advertising ;)
Kc = [Ç]ç.[D]d
[THE]The.[B]B
In the expression of Kc, we never use solid participants or liquid water, as they are constant items in the reaction.
See some examples:
Example 1: Ammonia Formation Balance
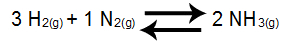
In the balance above, the product is NH3, which has a coefficient of 2, and the reactants are H2 and the N2, which present, respectively, the coefficients 3 and 1. All equilibrium participants are gaseous, so they can be part of Kc. From these data, the Kc expression will be:
Kc = [NH3]2
[H2]3.[N2]1
Example 2: Balance of calcium carbonate decomposition
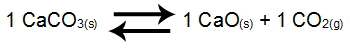
In the balance above, the products CaO and the CO2and the reagent CaCO3have coefficient 1. How CaCO3 and CaO are solid, they cannot be part of Kc. From these data, the equilibrium Kc expression will be:
Kc = [CO2]1
NOTE: The expression will have no denominator because the reagent is solid. So, we leave the expression without denominator or we put the number 1 in the denominator (number that indicates the constancy of a participant).
Example 3: Balance of ammonium chloride formation
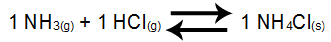
In the above equilibrium, the NH reagents3 and the HCl and the NH product4Cl have coefficient 1. As the participant of the NH balance4Cl is solid, it cannot be part of Kc. From these data, the equilibrium Kc expression will be:
Kc = 1
[NH3]1.[HCl]1
NOTE: As we do not have any balance participant in the numerator, it is necessary to place the number 1 (number that indicates the constancy of a participant).
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "What is Kc?"; Brazil School. Available in: https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-kc.htm. Accessed on June 28, 2021.
What is Chemistry?

Understand what Kp is, the equilibrium constant in terms of pressure, and know how to obtain it by using pressures partials of all gases present in a chemical equilibrium, which can be in atmospheres (atm) or millimeters of mercury (mmHg). Click here and find out more about this subject!
Chemistry

Test your knowledge and learn more with this list of solved exercises on chemical balances. Through this material, you will be able to better understand how to work equilibrium constants (Kp, Kc and Ki), equilibrium shift, pH and pOH, as well as equilibrium in so-called buffer solutions.

