In house cleaning, in many situations, it is possible to use vinegar to eliminate bad smells. But does it end up with all sorts of unpleasant odors? In what situations can it be used?
To understand how vinegar eliminates bad odors, we first need to understand what constitutes these odors. The smells that cause us an unpleasant feeling are composed of molecules that have several organic functions. See some examples:
* carboxylic acids: have 1 to 3 carbons and have a strong to irritating odor; acids with 4 to 10 carbons have an extremely unpleasant odor, with rancid and pungent smells. Some examples are:

* sulfur compounds or thiocompounds: Several sulfur-containing compounds are "stinky". They are usually derived from hydrogen sulfide gas, H2S, which is responsible for the rotten egg smell.
Many sulfur compounds, especially from the thiol group, also called mercaptans, are added to cooking gas because they have an extremely unpleasant odor and can be readily detected by the consumer even at low concentrations. This alert reduces the risk of accidents. Generally, among the thiols used are ethanethiol, butan-1-thiol and 1,1-dimethylethanethiol shown below:
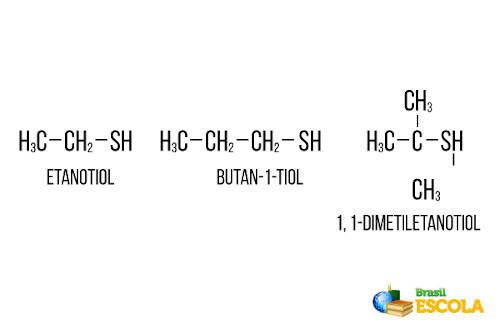
In addition to these, we also have methanethiol (CH3 ─ S ─ H), which is considered the compost with one of the worst smells in the world.
* Nitrogen aromatic compounds:
To give you an idea, the skatole (C9H9N) it's an aromatic amine responsible for the smell of feces.
Amines are also present in unpleasant odors because many of them are produced in the decomposition of living things. The unpleasant smell characteristic of rotten fish, for example, is due to trimethylamine and also to pyridine (aromatic amine):
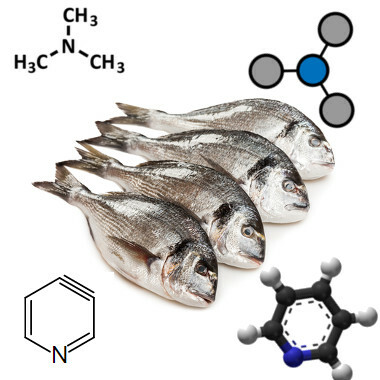
Trimethylamine and pyridine are responsible for the smell of rotten fish
Now let's talk about the vinegar. It consists of an aqueous solution of about 4% by volume of Acetic Acid. This organic compound, which is also called ethanoic acid, belongs to the carboxylic acid family, and its structure is shown below:

Structural formula of ethanoic or acetic acid
In order for the vinegar to eliminate bad smells, it is necessary that a neutralization reaction, in which substances that do not have the bad odor are produced. since all neutralization reaction occurs between an acid and a base, vinegar, which is an acid, effectively eliminates only basic odors.
The nitrogen compounds mentioned are alkaline, that is, they have a basic character (pH > 7). According to the Lewis acid-base theory, a base is any chemical species capable of offering a pair of electrons. But according to the Bronsted-Lowry theory, base is every chemical species capable of receiving a proton (H+).
Do not stop now... There's more after the advertising ;)
Thus, nitrogen compounds, such as aromatic amines, act as bases because nitrogen has a pair of electrons that it can supply to another chemical species or it can receive a proton (H+).
Vinegar can then be used to eliminate the fishy smell, as the following reaction occurs between acetic acid and pyridine:
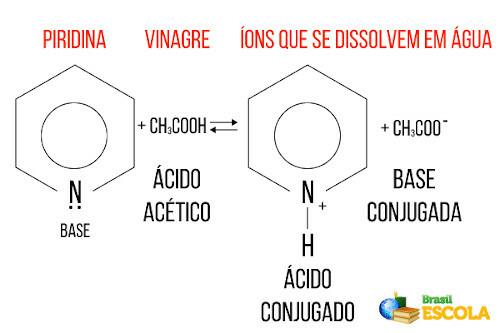
Note that the pyridine acted as a base, as it received a proton from acetic acid from the vinegar, formed ions that increased its solubility in water, and eliminated the rotten fish odor.
So, in everyday life, you can wash your hands with vinegar and then with water to finish the fish smell that stays in the hands. This can also be done to end the smell of fetid grease and with that rancid smell and taste of chicken.
Vinegar also acts in a similar way to get rid of the musty smell on clothes. You can soak the clothes in a bowl of water and vinegar.
Another application of vinegar is to eliminate the smell of urine and feces of dogs and cats. After you remove these residues, you can wipe the area with a cloth soaked in a solution made up of 2/3 warm water and 1/3 vinegar. Finally, apply some of the pure product over the area and let it dry naturally.
But according to the reactions seen, vinegar is not effective in eliminating, for example, the odor of sweat (bromhidrosis), for, as stated, this unpleasant smell is due to carboxylic acids.
Sweat is secreted by our eccrine and apocrine glands, consisting basically of water (99%), sodium chloride, low molar mass carboxylic acids, urea, salts of iron, potassium, ammonium, lactic acid, proteins and others components.
It is then eliminated by the sweat glands, initially without any unpleasant odor. However, in our skin, there are bacteria that metabolize sweat substances and produce some bad smelling compounds such as butyric acid, caproic acid and others associated with amines and mercaptans.
So, to get rid of underarm odor, it is more recommended to use a basic substance such as milk of magnesia (magnesium hydroxide solution, Mg(OH)2), which will make the environment basic, causing the death of bacteria and, consequently, the decomposition of organic substances eliminated in sweat. Most deodorants even work because they have an active component that inhibits the growth of bacteria, such as triclosan.
By Jennifer Fogaça
Graduated in Chemistry