The structural formula is a way of representing the bonds between the elements, with each pair of electrons shared between two atoms being symbolized by a dash:
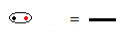
Shared electron pair symbolized by a dash
This means that all types of covalent bonds appear in the structural formula, whether single, double or triple:
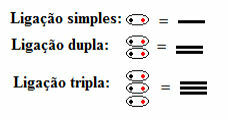
Single, double and triple bonds symbolized by dashes in structural formulas
Look at some examples of small molecule structural formulas involving few bonds and notice how shared electron pairs are represented.
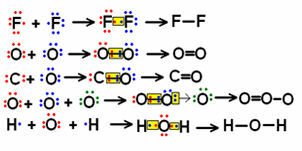
Examples of structural formulas for some molecules
The formulas shown above left, where electronic pairs are symbolized by “dots”, are called electronic formulas or Lewis formulas. All of these molecules are inorganic substances, but structural formulas are generally more used in the case of organic compounds, which are those formed by the element carbon, which are not of mineral origin (as in the case of carbon monoxide (CO), which is considered inorganic).
Since carbon is tetravalent (it makes four covalent bonds), it has the large ability to bind to different atoms and other carbons, forming an infinity of chains carbonic. That's why the structural formula is important because
indicates the arrangement of atoms in the chain.To understand, consider the following: a molecular formula indicates only the number of each element in a molecule of the substance. For example, let's say we have the molecular formula C3H6, we know then that it has three carbon atoms and six hydrogen atoms, but how are they bonded? The structural formula will tell us this and we can actually find out which compound it is. Note below that this molecular formula can give rise to two different structural formulas and, consequently, to two distinct substances:

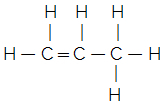
Cyclopropane Propene
These can be calledflat structural formulas, in which all connections and all elements are drawn on the plane of the paper, the blackboard, the computer screen, etc.
Do not stop now... There's more after the advertising ;)
However, many carbon chains are very large and quite complex, so it would be difficult to have to write the structural formula flat every time.
Thus, other forms of representation of the structure of molecules were created that are more simplified. The first is the simplified or condensed structural formula, in which the amount of hydrogen atoms attached to each carbon is abbreviated by putting its symbol (H) just once and adding in the lower right corner an index, which is a number that shows how many hydrogens there is.
For example, consider the flat structural formula of common ether:
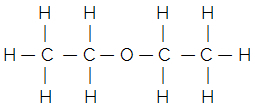
Its simplified or condensed structural formula is given by:
H3C — CH2 — O — CH2 — CH3
Much simpler, isn't it?!
But knowing that carbon always makes four bonds and that hydrogen only makes one bond, another even simpler formula emerged, which is the formula of dashes. If you wish, you can see how to write it in more detail in the text Molecular Formulas of Organic Compounds. But basically, in this formula, the groups C, CH, CH are omitted2 and CH3, which are represented by zigzag-connected dashes. Below we have the structural formula of the aforementioned ether:
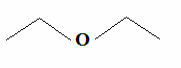
Common Ether Trace Formula
It is quite true, however, that substance formulas are not exactly flat in space. Therefore, in some cases, to give a more spatial idea, they write formulas in perspective, where links can indicate whether the atom is in the plane (normal stroke), behind the plane (dotted wedge) or in front of the plane (full wedge):

Representations in formulas in perspective
For example, look at the cortisol formula below. Note that one hydrogen, one hydroxyl group (OH) and two methyl groups (CH3 – which are omitted) are ahead of the plane, while two hydrogens and a hydroxyl group are behind the plane, and the rest is in the plane.

Structural formula in cortisol perspective
By Jennifer Fogaça
Graduated in Chemistry
Chemical formulas, flat structural formula, Couper structural formula, triple bond, gas nitrogen, electronic formula, Lewis formula, molecular formula, single bond, double bond, gas carbonic.