radioactivity law, or laws of radioactivity, is a set of norms or events discovered by soddy and Fajans. These two scientists developed the laws that explain the transformations undergone by an unstable nucleus of an atom after emitting alpha radiation or beta radiation.
Note: A gamma radiation does not appear in the description of these laws because it is a electromagnetic wave, therefore, does not involve nuclear particles.
According to these scientists, there are two radioactivity laws, a specific one for the alpha radiation and another for the beta radiation, which are discussed below.
First law of radioactivity
According to Soddy and Fajans, when the nucleus of a radioactive atom emits alpha radiation, it always forms a new atom whose nucleus contains two protons and two neutrons less than the parent atom.
The loss of these particles from the nucleus of the atom of origin causes the new nucleus to present a atomic number two units smaller and a mass number four units smaller.
This event can be represented by the following general equation:

Equation representing the first law of radioactivity
Example: Suppose a uranium 238 atom emits alpha radiation from inside its nucleus.
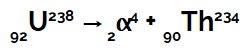
Chemical equation representing uranium emitting alpha radiation
We can see that when the uranium atom (mass number 238 and atomic number 92) emits alpha radiation (mass number 4 and atomic number 2), forms a new nucleus, of thorium, which has mass number 234 and number atomic 90.
Do not stop now... There's more after the advertising ;)
Second law of radioactivity
According to this radioactivity law, when the nucleus of a radioactive atom emits beta radiation, it always forms a new atom whose nucleus contains the same mass number, but with an atomic number one more unit than the atom of origin.
This event can be represented by the following general equation:
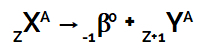
Equation representing the second law of radioactivity
Example: When a carbon 14 atom emits beta radiation from the interior of its nucleus:
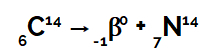
Chemical equation representing carbon emitting beta radiation
We can see that when a carbon atom (mass number 14 and atomic number 6) emits beta radiation (mass number 0 and atomic number -1), forms a new nucleus, of nitrogen, which has mass number 14 and number atomic 7.
This is because, according to scientist Henrico Fermi, a neutron present in the nucleus undergoes a transmutation, transforming itself into a proton, a neutrino and an electron. The electron and neutrino leave the nucleus, and the proton stays in the nucleus.
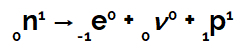
Equation representing neutron transmutation
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "What is the law of radioactivity?"; Brazil School. Available in: https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-lei-radioatividade.htm. Accessed on June 27, 2021.