specific heat and the amount of heat necessary for each gram of a substance to undergo a variation of temperature corresponding to 1°C. This quantity is a characteristic of each type of substance and indicates the behavior of the material when exposed to a heat source.
The table below indicates the specific heat of some substances.

applications
In the study of Calorimetry, the specific heat is present in the mathematical definition of sensible heatand of the thermal capacity of a material. Some everyday phenomena can be better understood from the definition of specific heat.
Note in the table above that the specific heat of sand is five times less than that of water. While each gram of sand needs only 0.2 cal to change its temperature by 1°C, water needs 1 cal to perform the same task. Here we understand why during the day the sand on the beach is at a higher temperature than the water.
Do not stop now... There's more after the advertising ;)
Mind Map: Specific Heat
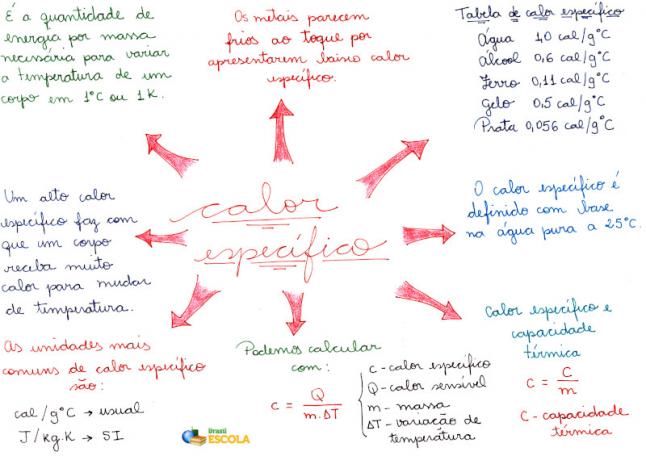
* To download the mind map in PDF, Click here!
Molar specific heat
For some substances it is appropriate to specify the amount of substance in mol. One mole of substance corresponds to the approximate amount of 6.02 x 1023 molecules. For the quantity of substance determined in mol, the molar specific heat of the substance is used.
The unit of measurement for the molar specific heat is cal/mol°C, that is, the amount of energy received by each mol of the substance for a temperature variation corresponding to 1 °C to occur.
Factors influencing specific heat
Intermolecular Forces: The larger theintermolecular bond strength of the substance, the greater the energy needed to break the bonds and effectively transform the material. Generally, substances that havehydrogen bonds in their structure they have high specific heat.
Impurities: Impurities present in different materials can change the substance's specific heat value.
Watch out!
Specific heat does not indicate the amount of heat needed for each gram or mole of a substance. increase your temperature by 1°C, but indicate the heat needed for the 1°C variation. This means that both increase and decrease in body temperature can occur. In the table above, thegold it is the material with the lowest specific heat, which means that this material is extremely sensitive to temperature variations. To heat or cool a certain amount of gold, little energy received or given up is required.
* Mind Map by Me. Rafael Helerbrock
By Joab Silas
Graduated in Physics
Would you like to reference this text in a school or academic work? Look:
JUNIOR, Joab Silas da Silva. "What is specific heat?"; Brazil School. Available in: https://brasilescola.uol.com.br/o-que-e/fisica/o-que-e-calor-especifico.htm. Accessed on June 27, 2021.

