Nitrocompound is an organic compound derived from the chemical reaction between nitric acid (HNO3) it is a alkane (open chain saturated hydrocarbon) or an aromatic. When nitric acid reacts with alkane or aromatic, a substitution reaction occurs in which the acid loses a hydroxyl group (OH), and the organic compound loses a hydrogen:
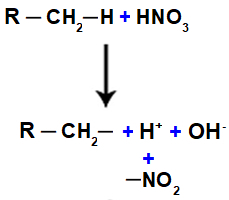
Next, we have the formation of a water molecule, resulting from the union between OH and H, while the NO group2 (which is left over from the acid) binds to the alkane or aromatic, forming the nitro compound.
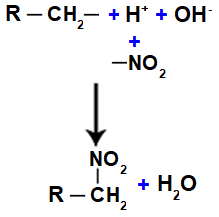
Representation of water and nitrocompound formation
Thus, the main structural feature of a nitro compound is the presence of one or more nitro groups (AT THE2) linked to an alkane or an aromatic.
properties
With respect to properties physical, we can highlight:
In general, they are viscous liquids at room temperature (with the exception of nitro compounds of low molar mass, which are fluid liquids);
They have high melting and boiling points;
They are denser than water;
In general, they are insoluble in water, with the exception of nitromethane and nitroethane;
When formed by an aliphatic chain, they have a pleasant aroma and are not poisonous. Now, if formed by an aromatic chain, they are poisonous and have an unpleasant aroma;
O type of intermolecular force that unites its molecules is the permanent dipole, as they have polar characteristics.
Regarding chemical properties, we must know that the nitro compounds they are very reactive, that is, they are used in various organic reactions, such as substitution reactions.
The official nomenclature rule, proposed by the IUPAC (International Union of Pure and Applied Chemistry), for nitro compounds é:
Do not stop now... There's more after the advertising ;)
Nitro + prefix + infix + o
Note: The prefix is related to the number of carbons present in the nitro compound chain. The infix is related to the type of bonds present between carbons.
Examples:
Nomenclature of a normal chain nitro compound

Initially, it is interesting to number the string of nitro compound (always from the carbon closest to the carbon that has the nitro group):
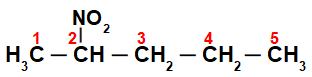
By numbering the string of the nitro compound, we have that in it there are five carbon atoms (prefix pent), just single bonds between the carbons (infix an) and the nitro group on carbon 2. The name of this structure is 2-nitropentane.
Nomenclature of a normal chain nitro compound
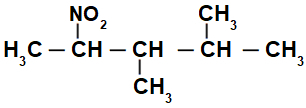
Structural formula of a branched nitro compound
Initially, it is interesting to number the string of nitro compound (always from the carbon closest to the carbon that has the nitro group) going to the end that has the highest number of carbons for the main chain:
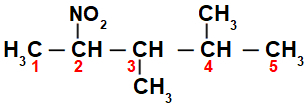
By numbering the string of the nitro compound, we have that in it there are 5 carbon atoms (prefix pent), only single bonds between the carbons (infix an), two methyl radicals (on carbon numbers 3 and 4) and the nitro group on carbon 2. The name of this structure is 3,4-dimethyl-2-nitropentane.
Uses
You nitro compounds, in general, they can be used in the manufacture of pesticides, dyes, aniline, bactericides, fungicides, additives, solvents; they also act as explosives and in oil refining.
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "What is nitrocompound?"; Brazil School. Available in: https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-nitrocomposto.htm. Accessed on June 28, 2021.

