geometric isomer is a kind of space isomerism which evaluates and compares the position in space of the ligands of two carbon atoms in a chain. This evaluation is made from an imaginary plane between the involved carbons.
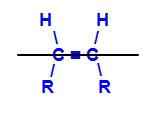
Imaginary plane dividing the molecule
The imaginary plane in the structure above divides the molecule into an upper plane and a lower plane. With this, we can evaluate and compare the carbon ligands involved in each of these planes. See the types of geometric isomers:
→ Cis-trans geometric isomer
A compound has cis-trans geometric isomerism when the chain displays:
Open chain with a double bond between two carbons, which have the same linkers to each other. See an example:
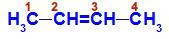
Structural formula of But-2-ene
In the structure, we can observe that both carbon 2 and carbon 3 have the same ligands, which are hydrogen (H) and methyl (CH3).
Closed chain with two carbons that have the same ligands to each other. See an example:
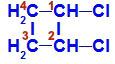
Structural formula of 1,2-dichloro-cyclobutane
In the structure, we can observe that both carbon 1 and carbon 2 have the same ligands, which are hydrogen (H) and chlorine (Cl).
To explain cis-trans isomerism, we will use but-2-ene:
a) Cis isomer: is the geometric isomer in which the same ligands occupy the same plane. In the example below, the hydrogens are on the same plane, as are the methyl radicals.

Structural formula of cis-but-2-ene
b) Trans isomer: is the geometric isomer in which different ligands occupy the same plane. In the example below, we have on the same plane a hydrogen and a methyl (CH3).
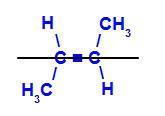
Structural formula of trans but-2-ene
Do not stop now... There's more after the advertising ;)
→ E-Z geometric isomer
A compound has E-Z geometric isomerism when the chain has:
Open chain with a double bond between two carbons, which have all or some different linkers. See an example:
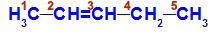
Structural formula of pent-2-ene
In the structure, we can see that carbon 2 has the methyl ligand (CH3) and hydrogen (H), and carbon 3 has the hydrogen (H) and ethyl (H) ligand3C-CH2).
Closed chain with two carbons that have all or some ligands different from each other. See an example:
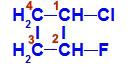
Structural formula of 1-chloro-2-fluoro-cyclobutane
In the structure, we can observe that carbon 1 has hydrogen and chlorine as the main ligands, and carbon 2 has hydrogen and fluorine as the main ligands.
NOTE: Among the different ligands, in geometric isomerism, we evaluate the ligand's complexity (number of atoms) or the atomic number.
To explain the E-Z isomer, we will use both pent-2-ene and 1-chloro-2-fluoro-cyclobutane:
a) E-isomer
geometric isomer where the more complex ligands or those with the highest atomic number are positioned on opposite planes. In the following example, the most complex ligand on carbon 2 is methyl, and the most complex ligand on carbon 3 is ethyl, which are positioned on different planes.
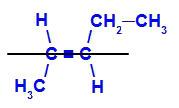
Structural formula of E-pent-2-ene
b) Isomer Z
geometric isomer where the more complex ligands or those with the highest atomic number are positioned on the same plane. In the following example, the highest atomic number ligand of carbon 1 is chlorine (Z = 17), and the highest atomic number ligand of carbon 2 is fluorine (Z = 9), which are positioned in the same plane.
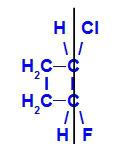
Structural formula of Z-1-chloro-2-fluoro-cyclobutane
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "What is geometric isomerism?"; Brazil School. Available in: https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-isomeria-geometrica.htm. Accessed on June 28, 2021.
Chemistry
Know what the various types of plane and spatial isomers are all about, such as function, position, chain, tautomerism, metamerism, cis-trans geometric and optical isomerism.
